
What is the absolute configuration of the following molecules? (NS = the molecule has no centres) Note: For the purpose of this question only, the order of stereocenters is not specified i.e., R, S = S, R.
I II III IV a. R R, S R NS b. R R, R S R, R c. R R, S NS NS d. R R, S R R, S

| I | II | III | IV | |
| a. | R | R, S | R | NS |
| b. | R | R, R | S | R, R |
| c. | R | R, S | NS | NS |
| d. | R | R, S | R | R, S |
Answer
511.8k+ views
Hint :CIP rules which stand for Cahn-Ingold-Prelog system is a set of rules which define the stereochemical configuration of any chiral carbon i.e., stereocenters with the help of designations rectus represented by ‘R’ which means right-handed and sinister represented by ‘S’ which means left-handed. This RS configuration given to the stereocenters of an organic compound is referred to as its absolute configuration.
Complete Step By Step Answer:
The two most important points to find absolute configuration are:
The compound must consist of a chiral carbon, it must have a stereocenter.
The priority is provided to the groups attached to the chiral carbon in increasing order of their atomic number.
The priorities given to groups along with their absolute configuration for the given compounds are as follows:
Compound-I:
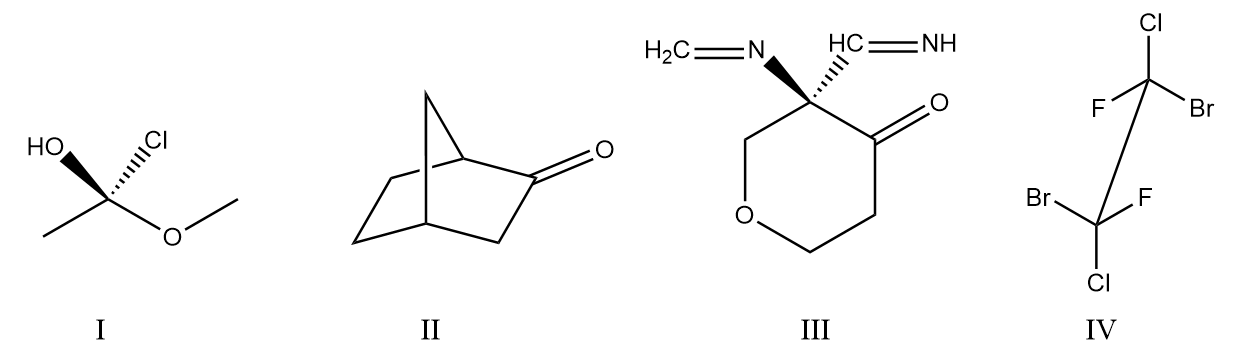
As the arrow is heading towards the clockwise direction, it must be an R configuration but the group with first priority is present below the plane and the group with fourth priority is present at the plane, so we need to rotate the bonds at the chiral centre.
Number of rotations $ = 2 $
So, the final configuration of the compound $ = R $
Compound-II:
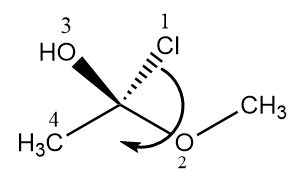
The second compound given is a bicyclic ring with two chiral centres. Let us check the absolute configuration of each chiral centre separately as follows:
At the first chiral centre, the arrow is heading in an anticlockwise direction so it is S configuration.
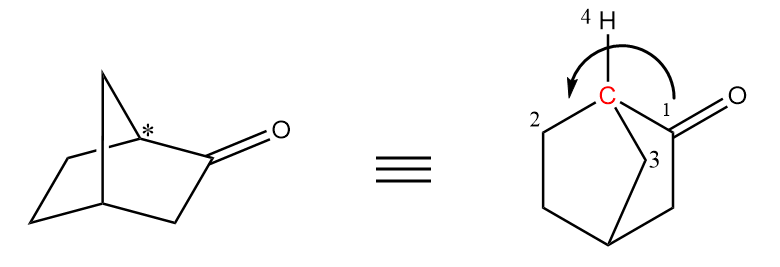
At the second chiral centre, the arrow is heading in a clockwise direction so it is R configuration.
Hence, the final absolute configuration of given molecule $ = R,\;S $
Compound-III:
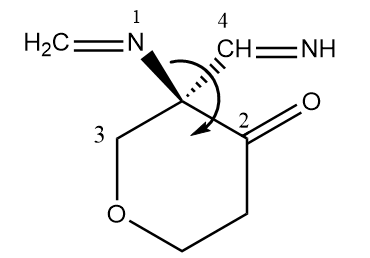
As the arrow is heading towards the clockwise direction and the fourth priority group is present below the plane, so the molecule is of R configuration.
Compound-IV:
The fourth compound consists of two chiral centres arranged in staggered form. So, first we need to arrange it in eclipsed form in order to check its configuration. The eclipsed form of the given compound is as follows:
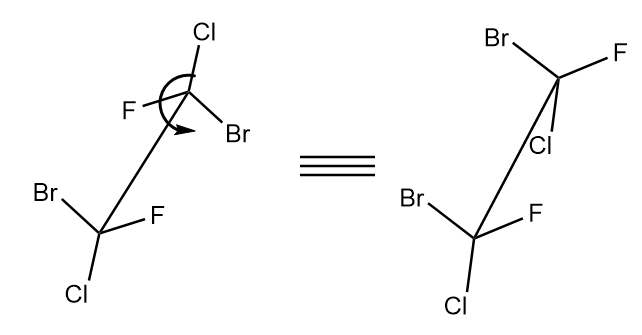
Now, let us check the absolute configuration of each chiral centre separately as follows:
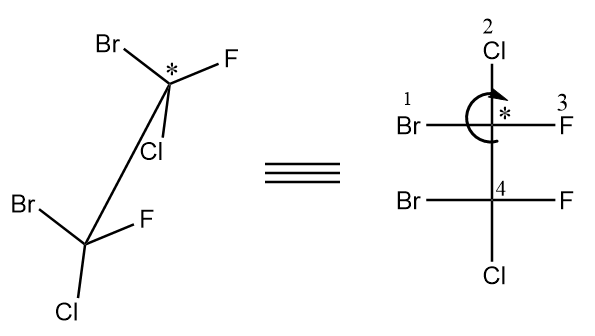
For the first chiral centre, the arrow is heading toward the clockwise direction so it is R configuration.
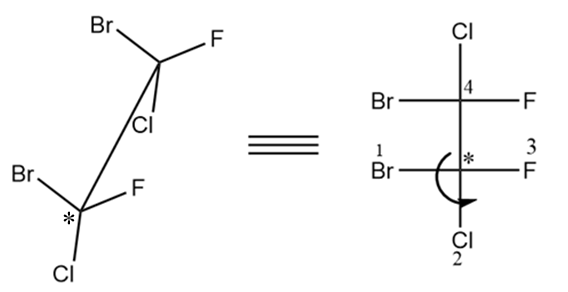
For the second chiral centre, the arrow is heading towards the anticlockwise direction, so it is of S configuration.
Hence the final configuration of the molecule $ = R,\;S $
Therefore, option (D) is the correct answer.
Note :
It is important to note that the group with fourth priority must be present below the plane while determining the absolute configuration of a molecule. If it is not present below the plane, then necessary rotation of bonds around the chiral centre are required and if the odd number of rotation of bonds takes place, then the inversion in configuration is observed.
Complete Step By Step Answer:
The two most important points to find absolute configuration are:
The compound must consist of a chiral carbon, it must have a stereocenter.
The priority is provided to the groups attached to the chiral carbon in increasing order of their atomic number.
The priorities given to groups along with their absolute configuration for the given compounds are as follows:
Compound-I:

As the arrow is heading towards the clockwise direction, it must be an R configuration but the group with first priority is present below the plane and the group with fourth priority is present at the plane, so we need to rotate the bonds at the chiral centre.
Number of rotations $ = 2 $
So, the final configuration of the compound $ = R $
Compound-II:
The second compound given is a bicyclic ring with two chiral centres. Let us check the absolute configuration of each chiral centre separately as follows:

At the first chiral centre, the arrow is heading in an anticlockwise direction so it is S configuration.
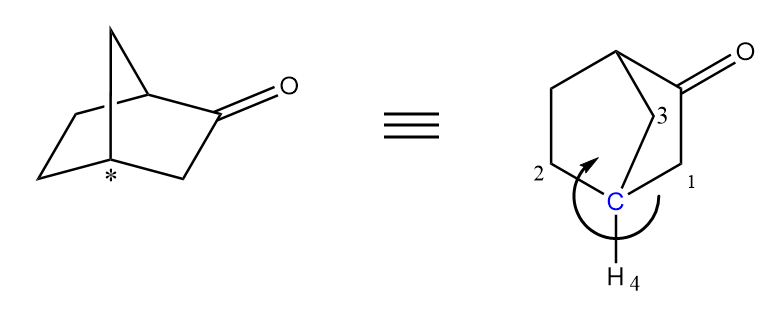
At the second chiral centre, the arrow is heading in a clockwise direction so it is R configuration.
Hence, the final absolute configuration of given molecule $ = R,\;S $
Compound-III:

As the arrow is heading towards the clockwise direction and the fourth priority group is present below the plane, so the molecule is of R configuration.
Compound-IV:
The fourth compound consists of two chiral centres arranged in staggered form. So, first we need to arrange it in eclipsed form in order to check its configuration. The eclipsed form of the given compound is as follows:

Now, let us check the absolute configuration of each chiral centre separately as follows:

For the first chiral centre, the arrow is heading toward the clockwise direction so it is R configuration.

For the second chiral centre, the arrow is heading towards the anticlockwise direction, so it is of S configuration.
Hence the final configuration of the molecule $ = R,\;S $
Therefore, option (D) is the correct answer.
Note :
It is important to note that the group with fourth priority must be present below the plane while determining the absolute configuration of a molecule. If it is not present below the plane, then necessary rotation of bonds around the chiral centre are required and if the odd number of rotation of bonds takes place, then the inversion in configuration is observed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




