
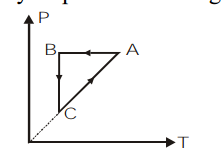
\[ABCA\] is a cyclic process. Its \[P-V\] graph would be
\[A)\]
\[B)\]
\[C)\]
\[D)\]





Answer
591.9k+ views
Hint: We will use the ideal gas relation \[PV\propto nT\] for analysis of all three processes given in the \[P-T\] graph and convert the data into \[P-V\] graph. The given process \[ABCA\] is a cyclic process but it is completing one cycle with 3 processes. So we will need to convert each process separately.
Complete step by step answer:
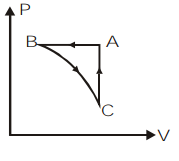
The given process \[ABCA\] completes one cycle by undergoing 3 cycles as \[BC\], \[CA\] and \[AB\]. We can plot the \[P-V\] graph of the given process by analyzing the given \[P-T\] graph individually for each process.
Let us take the first process \[BC\] on the \[P-T\] graph. We can observe that the temperature remains constant for this process and pressure is decreasing. So by the ideal gas equation, at constant temperature, \[P \propto \dfrac{1}{V}\]. So as the pressure is decreasing, the volume will increase.
Now, let us take the process \[CA\] on the \[P-T\] graph. Here, we can observe the temperature and pressure increasing as per the ideal gas relation $P \propto T$. But this relation is valid only at constant volume. So in the \[P-V\] graph there will be no change in volume.
Then, we will consider the last process in this cyclic process which is \[AB\]. If we look at the \[P-T\] graph of this process we can understand that pressure remains constant and temperature is decreasing. So, according to the ideal gas relation, at constant pressure, \[V\propto T\]. That means the volume will decrease. So in the \[P-V\] graph the pressure remains constant and volume will decrease.
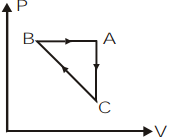
So, we can plot the \[P-V\] graph of the given cyclic process \[ABCA\] as,
So, the correct answer is option c.
Note:
We can solve this question only by using the relationship between the parameters given by the ideal gas equation. But we can solve this question quickly by just analyzing each process and the variations occurring in that process and converting it to the desired plot if we know the relation between P, V and T according to the ideal gas relation.
Complete step by step answer:
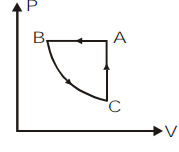
The given process \[ABCA\] completes one cycle by undergoing 3 cycles as \[BC\], \[CA\] and \[AB\]. We can plot the \[P-V\] graph of the given process by analyzing the given \[P-T\] graph individually for each process.
Let us take the first process \[BC\] on the \[P-T\] graph. We can observe that the temperature remains constant for this process and pressure is decreasing. So by the ideal gas equation, at constant temperature, \[P \propto \dfrac{1}{V}\]. So as the pressure is decreasing, the volume will increase.
Now, let us take the process \[CA\] on the \[P-T\] graph. Here, we can observe the temperature and pressure increasing as per the ideal gas relation $P \propto T$. But this relation is valid only at constant volume. So in the \[P-V\] graph there will be no change in volume.
Then, we will consider the last process in this cyclic process which is \[AB\]. If we look at the \[P-T\] graph of this process we can understand that pressure remains constant and temperature is decreasing. So, according to the ideal gas relation, at constant pressure, \[V\propto T\]. That means the volume will decrease. So in the \[P-V\] graph the pressure remains constant and volume will decrease.
So, we can plot the \[P-V\] graph of the given cyclic process \[ABCA\] as,

So, the correct answer is option c.
Note:
We can solve this question only by using the relationship between the parameters given by the ideal gas equation. But we can solve this question quickly by just analyzing each process and the variations occurring in that process and converting it to the desired plot if we know the relation between P, V and T according to the ideal gas relation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




