
a) Write the structure of main products when aniline reacts with the following reagents
i.$B{{r}_{2}}$ water
ii.$HCl$
iii.${{(C{{H}_{3}}CO)}_{2}}/pyridine$
b) Arrange the following in increasing order of their boiling point. ${{C}_{2}}{{H}_{5}}N{{H}_{2}},{{C}_{2}}{{H}_{5}}OH,{{(C{{H}_{3}})}_{3}}N$
c) Give a simple chemical test to distinguish between the following pair of compound
${{(C{{H}_{3}})}_{2}}NH$ and ${{(C{{H}_{3}})}_{3}}N$
Answer
548.4k+ views
Hint: The aniline has a structure in which an amine group is attached to the benzene ring. The amine group activates the ring as it has lone pairs of electrons which will take part in the resonance.
The electrophiles would get attached to the ortho- and para- positions because of higher availability of electron clouds in those parts of the ring.
Complete step-by-step answer:
We will be answering each part and subpart of this question is a systematic manner.
a)As the first part concerns mainly about the reactivity of aniline, we will at first consider the structure of aniline.
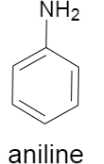
As we can see it contains an amine group attached to the benzene ring. Now, we will consider each of the reactants which would react to this structure, in the subparts.
i. Bromine or $B{{r}_{2}}$ water
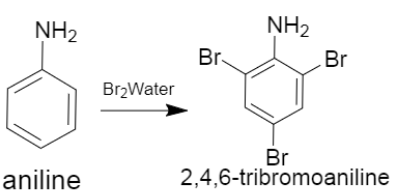
As we can see the reaction of the aniline with bromine water gives $2,4,6-$ tribromoaniline as a product.
ii.In the next part, we are asked to determine the product of reaction which occurs between aniline and hydrochloric acid. The reaction is shown below,
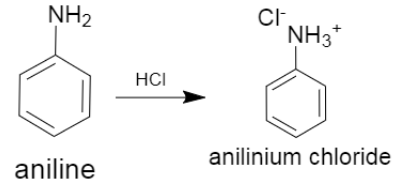
The product which is formed in this reaction is termed as anilinium chloride.
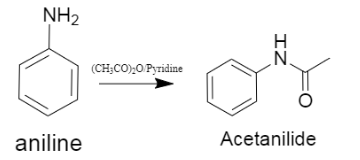
iii.In this we are supposed to predict the reaction between acetic anhydride and aniline, which gives a product called acetanilide. The reaction is shown below.
Now we will move to the part (b) of the question.
b) We are asked to arrange some of the compounds in the increasing order of their boiling points. Now, one of them is ethanol which has the formula ${{C}_{2}}{{H}_{5}}OH$ which is a type of alcohol. And the other two are amines. We know that the alcohols have higher boiling point than amines. So, it will have the higher boiling point. Now we need to figure out which one among the two amines have higher boiling point. One of the amines is tertiary amine which has the formula ${{(C{{H}_{3}})}_{3}}N$ and the other one is ${{C}_{2}}{{H}_{5}}N{{H}_{2}}$ which is a type of primary amine. Since the tertiary amine does not have any hydrogen in it, which is attached to the nitrogen, there is absence of hydrogen bonding in tertiary amines. Unlike the case of primary amine which has hydrogens and so it experiences hydrogen bonding, and hence have a higher boiling point than the tertiary amine. So, the order becomes
${{(C{{H}_{3}})}_{3}}N<{{C}_{2}}{{H}_{5}}N{{H}_{2}}<{{C}_{2}}{{H}_{5}}OH$ which is the required answer.
c) Now, we will discuss a distinction test between a secondary and tertiary amines. The Hinsberg test can be used to distinguish between the two. It is based on the formation of sulfonamide. In this test, an amine reacts along with the reagent benzene sulfonyl chloride. If the formation of a product takes place, the amine would be of either a primary or secondary nature, as tertiary amines don't form stable sulfonamides. The reaction is as follows,
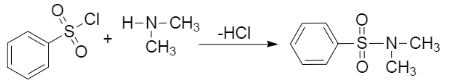
Benzene sulfonyl chloride
As we can see the secondary amine reacts with the reagent but the tertiary amine would not.
Note: The boiling point of alcohols are much higher than the boiling points of amines. And the boiling point of primary and secondary amines is higher than the boiling point of the tertiary amines because of the presence of hydrogen bonding in the former.
Hinsberg test is used to differentiate between the primary and tertiary amines, as tertiary amines would not react with the hinsberg reagent due to unavailability of hydrogens.
The electrophiles would get attached to the ortho- and para- positions because of higher availability of electron clouds in those parts of the ring.
Complete step-by-step answer:
We will be answering each part and subpart of this question is a systematic manner.
a)As the first part concerns mainly about the reactivity of aniline, we will at first consider the structure of aniline.

As we can see it contains an amine group attached to the benzene ring. Now, we will consider each of the reactants which would react to this structure, in the subparts.
i. Bromine or $B{{r}_{2}}$ water

As we can see the reaction of the aniline with bromine water gives $2,4,6-$ tribromoaniline as a product.
ii.In the next part, we are asked to determine the product of reaction which occurs between aniline and hydrochloric acid. The reaction is shown below,

The product which is formed in this reaction is termed as anilinium chloride.
iii.In this we are supposed to predict the reaction between acetic anhydride and aniline, which gives a product called acetanilide. The reaction is shown below.

Now we will move to the part (b) of the question.
b) We are asked to arrange some of the compounds in the increasing order of their boiling points. Now, one of them is ethanol which has the formula ${{C}_{2}}{{H}_{5}}OH$ which is a type of alcohol. And the other two are amines. We know that the alcohols have higher boiling point than amines. So, it will have the higher boiling point. Now we need to figure out which one among the two amines have higher boiling point. One of the amines is tertiary amine which has the formula ${{(C{{H}_{3}})}_{3}}N$ and the other one is ${{C}_{2}}{{H}_{5}}N{{H}_{2}}$ which is a type of primary amine. Since the tertiary amine does not have any hydrogen in it, which is attached to the nitrogen, there is absence of hydrogen bonding in tertiary amines. Unlike the case of primary amine which has hydrogens and so it experiences hydrogen bonding, and hence have a higher boiling point than the tertiary amine. So, the order becomes
${{(C{{H}_{3}})}_{3}}N<{{C}_{2}}{{H}_{5}}N{{H}_{2}}<{{C}_{2}}{{H}_{5}}OH$ which is the required answer.
c) Now, we will discuss a distinction test between a secondary and tertiary amines. The Hinsberg test can be used to distinguish between the two. It is based on the formation of sulfonamide. In this test, an amine reacts along with the reagent benzene sulfonyl chloride. If the formation of a product takes place, the amine would be of either a primary or secondary nature, as tertiary amines don't form stable sulfonamides. The reaction is as follows,

Benzene sulfonyl chloride
As we can see the secondary amine reacts with the reagent but the tertiary amine would not.
Note: The boiling point of alcohols are much higher than the boiling points of amines. And the boiling point of primary and secondary amines is higher than the boiling point of the tertiary amines because of the presence of hydrogen bonding in the former.
Hinsberg test is used to differentiate between the primary and tertiary amines, as tertiary amines would not react with the hinsberg reagent due to unavailability of hydrogens.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




