
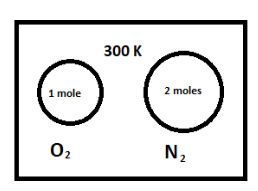
A vessel contains a mixture of one mole of oxygen and two moles of nitrogen at 300 K. The ratio of the average rotational kinetic energy per ${O_2}$ molecules to that per ${N_2}$ molecules is
A. $1: 1$
B. $1: 2$
C. $2: 1$
D. Depends on the moment of inertia of the two molecules
Answer
568.5k+ views
Hint: To find the ratio of the average rotational kinetic energy per molecule of the oxygen and nitrogen present in the mixture, we will use the law of equipartition of energy stating the equal distribution of energy among the degrees of freedom of the respective molecules. The degrees of freedom can be calculated using the formula:
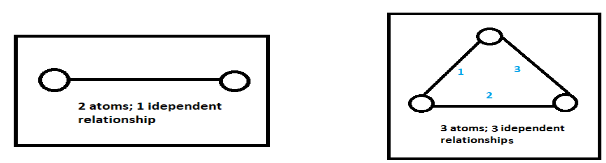
${D_f} = 3N - K$ where $N$ is the number of particles and $K$ is the independent relations among these particles.
Complete step by step answer:
It is given that we have one mole of oxygen $\left( {{O_2}} \right)$ gas and two moles of nitrogen \[\left( {{N_2}} \right)\] gas at the temperature $300 K$.
Now, according to the law of equipartition of energy:
When a system is in equilibrium (thermal), the total energy of the system is divided equally among all the degrees of freedom. Thus, the average rotational kinetic energy will also be divided equally among all the degrees of freedom for the moles of both the gases.
This average energy is given as:
$E = \dfrac{1}{2}KT....(1)$ where K is the Boltzmann’s constant and T is the temperature.
The temperature is constant for this system, the system will be in thermal equilibrium and this energy will be dependent on the degrees of freedom of respective gases. The degrees of freedom can be calculated using the formula ${D_f} = 3N - K$ where $N$ is the number of particles and $K$ is the independent relations among these particles.
For ${O_2}$ molecules, the degree of freedom can be calculated as:
${D_f} = 3N - K$ here,
Number of molecules (N) = 2
Independent relations among them (K) = 1 (known)
$
{D_{{f_{{O_2}}}}} = 3 \times 2 - 1 \\
\Rightarrow {D_{{f_{{O_2}}}}} = 6 - 1 \\
\Rightarrow {D_{{f_{{O_2}}}}} = 5 \\ $
For ${N_2}$ molecules, the degree of freedom can be calculated as:
${D_f} = 3N - K$ here,
Number of molecules (N) = 2
Independent relations among them (K) = 1 (known)
$
{D_{{f_{{N_2}}}}} = 3 \times 2 - 1 \\
\Rightarrow {D_{{f_{{N_2}}}}} = 6 - 1 \\
\Rightarrow {D_{{f_{{N_2}}}}} = 5 \\ $
The ratio of average energy of the molecules will be equal to the ratio of their degrees of freedom as the energy is divided equally amongst them. So the required ratio for both the gases is given as:
$\dfrac{{{E_{{O_2}}}}}{{{E_{{N_2}}}}} = \dfrac{{{D_{{f_{{O_2}}}}}}}{{{D_{{f_{{N_2}}}}}}}$
Substituting the values, we get:
$
\dfrac{{{E_{{O_2}}}}}{{{E_{{N_2}}}}} = \dfrac{1}{1} \\
\therefore {E_{{O_2}}}:{E_{{N_2}}} = 1:1 \\ $
Therefore, the ratio of the average rotational kinetic energy per ${O_2}$ molecules to that per ${N_2}$ molecules is 1: 1 and the correct option is A.
Note: The independent relations K among the molecules is generally known for monoatomic (having one atom), diatomic (having 2 atoms) and triatomic gases (having 3 atoms) as 0, 1 and 3 respectively. But, we can also find by drawing the number of molecules and finding the independent relations if forgotten as: 1 atom cannot have an independent relationship with any other atom, so it’s count is 0.
${D_f} = 3N - K$ where $N$ is the number of particles and $K$ is the independent relations among these particles.
Complete step by step answer:
It is given that we have one mole of oxygen $\left( {{O_2}} \right)$ gas and two moles of nitrogen \[\left( {{N_2}} \right)\] gas at the temperature $300 K$.
Now, according to the law of equipartition of energy:
When a system is in equilibrium (thermal), the total energy of the system is divided equally among all the degrees of freedom. Thus, the average rotational kinetic energy will also be divided equally among all the degrees of freedom for the moles of both the gases.
This average energy is given as:
$E = \dfrac{1}{2}KT....(1)$ where K is the Boltzmann’s constant and T is the temperature.
The temperature is constant for this system, the system will be in thermal equilibrium and this energy will be dependent on the degrees of freedom of respective gases. The degrees of freedom can be calculated using the formula ${D_f} = 3N - K$ where $N$ is the number of particles and $K$ is the independent relations among these particles.

For ${O_2}$ molecules, the degree of freedom can be calculated as:
${D_f} = 3N - K$ here,
Number of molecules (N) = 2
Independent relations among them (K) = 1 (known)
$
{D_{{f_{{O_2}}}}} = 3 \times 2 - 1 \\
\Rightarrow {D_{{f_{{O_2}}}}} = 6 - 1 \\
\Rightarrow {D_{{f_{{O_2}}}}} = 5 \\ $
For ${N_2}$ molecules, the degree of freedom can be calculated as:
${D_f} = 3N - K$ here,
Number of molecules (N) = 2
Independent relations among them (K) = 1 (known)
$
{D_{{f_{{N_2}}}}} = 3 \times 2 - 1 \\
\Rightarrow {D_{{f_{{N_2}}}}} = 6 - 1 \\
\Rightarrow {D_{{f_{{N_2}}}}} = 5 \\ $
The ratio of average energy of the molecules will be equal to the ratio of their degrees of freedom as the energy is divided equally amongst them. So the required ratio for both the gases is given as:
$\dfrac{{{E_{{O_2}}}}}{{{E_{{N_2}}}}} = \dfrac{{{D_{{f_{{O_2}}}}}}}{{{D_{{f_{{N_2}}}}}}}$
Substituting the values, we get:
$
\dfrac{{{E_{{O_2}}}}}{{{E_{{N_2}}}}} = \dfrac{1}{1} \\
\therefore {E_{{O_2}}}:{E_{{N_2}}} = 1:1 \\ $
Therefore, the ratio of the average rotational kinetic energy per ${O_2}$ molecules to that per ${N_2}$ molecules is 1: 1 and the correct option is A.
Note: The independent relations K among the molecules is generally known for monoatomic (having one atom), diatomic (having 2 atoms) and triatomic gases (having 3 atoms) as 0, 1 and 3 respectively. But, we can also find by drawing the number of molecules and finding the independent relations if forgotten as: 1 atom cannot have an independent relationship with any other atom, so it’s count is 0.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




