
A unit of $BaC{l_2}$(fluorite structure) is made up of:
(A) four $B{a^{2 + }}$ions and four $C{l^ - }$ions
(B) four $B{a^{2 + }}$ions and eight $C{l^ - }$ions
(C) eight $B{a^{2 + }}$ions and four $C{l^ - }$ions
(D) four $B{a^{2 + }}$ions and six $C{l^ - }$ions
Answer
577.5k+ views
Hint: Crystalline barium chloride is an orthogonal crystal structure and dehydrate $BaC{l_2}$ has a monocyclic crystal structure. Barium chloride is used industrially in the purification process of brine solution.
Complete step by step solution:
(1) Barium chloride is an inorganic salt.
(2) It is made up of barium cations and chloride anions.
(3) It is a unit solid which is water soluble.
(4) The molecular weight of barium chloride is $BaC{l_2}.$
(5) The melting point is ${962^\circ }C.$
(6) The structure of barium sulphide is
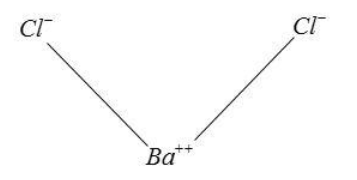
(7) It is obtained into two steps.
Step-1: From the barium surface at high temperature.
$BaS{O_4} + 4C \to BaS + 4CO$
Step-2: From barium sulphide.
$BaS + 2HCl \to BaC{l_2} + {H_2}S$
(8) Aqueous solutions of barium chloride have neutral $pH$ values since they contain cation of strong base and anion of strong acid.
Note:
1. Do not forget to balance the chemical equation you write. Balanced chemical equations show the number of units of a compound used in the reaction.
2. Barium chloride is used in the purification of brine solutions that are used in carrying chlorine plants.
Complete step by step solution:
(1) Barium chloride is an inorganic salt.
(2) It is made up of barium cations and chloride anions.
(3) It is a unit solid which is water soluble.
(4) The molecular weight of barium chloride is $BaC{l_2}.$
(5) The melting point is ${962^\circ }C.$
(6) The structure of barium sulphide is

(7) It is obtained into two steps.
Step-1: From the barium surface at high temperature.
$BaS{O_4} + 4C \to BaS + 4CO$
Step-2: From barium sulphide.
$BaS + 2HCl \to BaC{l_2} + {H_2}S$
(8) Aqueous solutions of barium chloride have neutral $pH$ values since they contain cation of strong base and anion of strong acid.
Note:
1. Do not forget to balance the chemical equation you write. Balanced chemical equations show the number of units of a compound used in the reaction.
2. Barium chloride is used in the purification of brine solutions that are used in carrying chlorine plants.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




