
A soap bubble of radius 2 cm is formed in another soap bubble of radius 6 cm.
Minimum possible radius with the pressure of this case is
A. \[\dfrac{5}{3}\,{\text{cm}}\]
B. \[\dfrac{7}{3}\,{\text{cm}}\]
C. \[\dfrac{4}{3}\,{\text{cm}}\]
D. \[\dfrac{3}{2}\,{\text{cm}}\]
Answer
565.8k+ views
Hint:The excess pressures inside each bubble will exert a pressure on one another. Recall the expression for the excess pressure inside a bubble and express it for both the bubbles. The net excess pressure will be the sum of the excess pressure inside both the bubbles.
Formula used:
Excess pressure, \[P = \dfrac{{4T}}{r}\]
Here, T is the surface tension and \[r\] is the radius of the soap bubble.
Complete step by step answer:
We have given the two soap bubbles such that one soap bubble of radius 2 cm is formed
inside the soap bubble of radius 6 cm. As we know, there is always an excess pressure inside
the soap bubble than the atmospheric pressure. These excess pressures inside each bubble
will exert a pressure on one another as shown in the figure below.
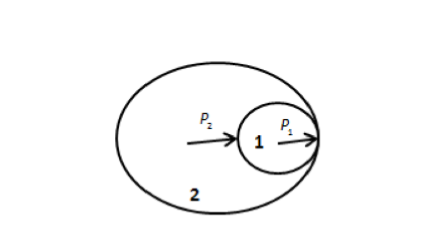
In the above figure, soap bubble 1 has excess pressure \[{P_1}\] and soap bubble 2 has
excess pressure \[{P_2}\].
Let’s express the excess pressure inside the smaller soap bubble as follows,
\[{P_1} = \dfrac{{4T}}{{{r_1}}}\] …… (1)
Here, T is the surface tension and \[{r_1}\] is the radius of the smaller soap bubble.
Let’s express the excess pressure inside the larger soap bubble as follows,
\[{P_2} = \dfrac{{4T}}{{{r_2}}}\] …… (2)
Here, \[{r_2}\] is the radius of the larger soap bubble.
The net excess pressure in this case will be,
\[P = {P_1} + {P_2}\]
\[ \Rightarrow \dfrac{{4T}}{r} = \dfrac{{4T}}{{{r_1}}} + \dfrac{{4T}}{{{r_2}}}\]
\[ \Rightarrow \dfrac{1}{r} = \dfrac{1}{{{r_1}}} + \dfrac{1}{{{r_2}}}\]
Substituting \[{r_1} = 2\,{\text{cm}}\] and \[{r_2} = 6\,{\text{cm}}\] in the above equation,
we get,
\[\dfrac{1}{r} = \dfrac{1}{2} + \dfrac{1}{6}\]
\[ \Rightarrow \dfrac{1}{r} = \dfrac{2}{3}\]
\[ \Rightarrow r = \dfrac{3}{2}\,{\text{cm}}\]
So, the correct answer is option (D).
Note:Note that the surface tension will be the same for both the soap bubbles. The smaller soap bubble will always be attached to the inner wall of the larger soap bubble. In this way, the larger soap bubble will exert a pressure on the smaller bubble. The pressure inside the soap bubble is always greater than the outside atmospheric pressure.
Formula used:
Excess pressure, \[P = \dfrac{{4T}}{r}\]
Here, T is the surface tension and \[r\] is the radius of the soap bubble.
Complete step by step answer:
We have given the two soap bubbles such that one soap bubble of radius 2 cm is formed
inside the soap bubble of radius 6 cm. As we know, there is always an excess pressure inside
the soap bubble than the atmospheric pressure. These excess pressures inside each bubble
will exert a pressure on one another as shown in the figure below.

In the above figure, soap bubble 1 has excess pressure \[{P_1}\] and soap bubble 2 has
excess pressure \[{P_2}\].
Let’s express the excess pressure inside the smaller soap bubble as follows,
\[{P_1} = \dfrac{{4T}}{{{r_1}}}\] …… (1)
Here, T is the surface tension and \[{r_1}\] is the radius of the smaller soap bubble.
Let’s express the excess pressure inside the larger soap bubble as follows,
\[{P_2} = \dfrac{{4T}}{{{r_2}}}\] …… (2)
Here, \[{r_2}\] is the radius of the larger soap bubble.
The net excess pressure in this case will be,
\[P = {P_1} + {P_2}\]
\[ \Rightarrow \dfrac{{4T}}{r} = \dfrac{{4T}}{{{r_1}}} + \dfrac{{4T}}{{{r_2}}}\]
\[ \Rightarrow \dfrac{1}{r} = \dfrac{1}{{{r_1}}} + \dfrac{1}{{{r_2}}}\]
Substituting \[{r_1} = 2\,{\text{cm}}\] and \[{r_2} = 6\,{\text{cm}}\] in the above equation,
we get,
\[\dfrac{1}{r} = \dfrac{1}{2} + \dfrac{1}{6}\]
\[ \Rightarrow \dfrac{1}{r} = \dfrac{2}{3}\]
\[ \Rightarrow r = \dfrac{3}{2}\,{\text{cm}}\]
So, the correct answer is option (D).
Note:Note that the surface tension will be the same for both the soap bubbles. The smaller soap bubble will always be attached to the inner wall of the larger soap bubble. In this way, the larger soap bubble will exert a pressure on the smaller bubble. The pressure inside the soap bubble is always greater than the outside atmospheric pressure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




