
A sample of gas expands from volume V1 to V2. The amount of work done by the gas is greatest when the expansion is
A)Isothermal
B)Adiabatic
C)Isobaric
D)All of these
Answer
585.6k+ views
Hint:To answer this question, you should recall the different types of thermodynamic processes. Plot a \[{\text{P}}\,\,{\text{v/s}}\,\,{\text{V}}\]graph of each process and calculate the area under the graph to compare the work in each process.
Complete Step by step solution:
We need to know about each of the thermodynamic processes mentioned in the aforementioned question. Taking a look at each of the options:
1).An isothermal process is a type of thermodynamic process in which the temperature change is zero. This is achieved when transfer of heat into or out of the system takes place very slowly such that thermal equilibrium is maintained.
2).An adiabatic process is the type of thermodynamic process in which there is no exchange of heat from the system to its surrounding. The heat exchange is zero both during expansion and compression.
3)An Isobaric process is the type of thermodynamic process where pressure change stays constant.
4).An Isochoric process is the type of thermodynamic process where the volume of the system remains constant, is called as isochoric process. For example: Heating of gas in a closed cylinder is an isochoric process.
Now we know about each thermodynamic process, we can plot the graph to calculate work done.
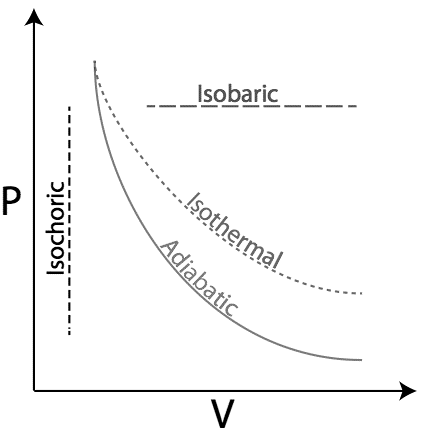
As we know that, the area under the \[{\text{P}}\,\,{\text{v/s}}\,\,{\text{V}}\] graph gives us the work done. From the above graph, we can see that the area is maximum under the Isobaric curve.
Hence, work done is maximum during an isobaric expansion curve.
Therefore, we can conclude that the correct answer to this question is option C.
Note: You should know about the gas laws governing these processes. These consist of three primary laws: Charles' Law, Boyle's Law and Avogadro's Law, which will later combine to form the General Gas Equation and Ideal Gas Law.
Complete Step by step solution:
We need to know about each of the thermodynamic processes mentioned in the aforementioned question. Taking a look at each of the options:
1).An isothermal process is a type of thermodynamic process in which the temperature change is zero. This is achieved when transfer of heat into or out of the system takes place very slowly such that thermal equilibrium is maintained.
2).An adiabatic process is the type of thermodynamic process in which there is no exchange of heat from the system to its surrounding. The heat exchange is zero both during expansion and compression.
3)An Isobaric process is the type of thermodynamic process where pressure change stays constant.
4).An Isochoric process is the type of thermodynamic process where the volume of the system remains constant, is called as isochoric process. For example: Heating of gas in a closed cylinder is an isochoric process.
Now we know about each thermodynamic process, we can plot the graph to calculate work done.

As we know that, the area under the \[{\text{P}}\,\,{\text{v/s}}\,\,{\text{V}}\] graph gives us the work done. From the above graph, we can see that the area is maximum under the Isobaric curve.
Hence, work done is maximum during an isobaric expansion curve.
Therefore, we can conclude that the correct answer to this question is option C.
Note: You should know about the gas laws governing these processes. These consist of three primary laws: Charles' Law, Boyle's Law and Avogadro's Law, which will later combine to form the General Gas Equation and Ideal Gas Law.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




