
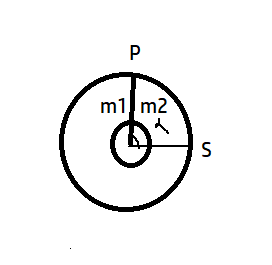
A ring shaped tube contains two ideal gases with equal masses and molar masses \[{{M}_{1}}\] =32 and \[{{M}_{2}}\]=28. The gases are separated by one fixed partition P and another movable stopper S which can move freely without friction inside the ring. The angle α in degree is
(A) 182
(B) 170
(C) 192
(D) 180

Answer
585.9k+ views
Hint: In this problem it is mentioned that masses of the gas in the ring are the same. Also, they are separated by fixed portions. Thus, the pressure exerted must be the same by both the gases.
Complete step by step answer:
Pressure exerted by gas A= pressure exerted by gas B
Using ideal gas law, PV=nRT
\[P=\dfrac{nRT}{V}\]
So \[{{P}_{1}}={{P}_{2}}\]
\[\dfrac{{{n}_{1}}RT}{{{V}_{1}}}=\dfrac{{{n}_{2}}RT}{{{V}_{2}}}\]here temperature of the both of them are same and R is a gas constant.
Let us find out the number of moles, it is given by the formula. \[n=\dfrac{m}{M}\]
Here m is the given mass and M is the molecular mass of the gas. It is given that the given mass for both of the gas are the same.
\[\dfrac{{{n}_{1}}}{{{V}_{1}}}=\dfrac{{{n}_{2}}}{{{V}_{2}}}\]
\[\Rightarrow \dfrac{32}{{{V}_{1}}}=\dfrac{28}{{{V}_{2}}}\]
\[\Rightarrow \dfrac{{{V}_{2}}}{{{V}_{1}}}=\dfrac{8}{7}\]------(1)
It is clear from the figure that total angle is \[{{360}^{0}}\] and gas 2 makes an angle \[\alpha \]. Since radius for both is same, thus,
\[\dfrac{{{V}_{2}}}{{{V}_{1}}}=\dfrac{\alpha }{360-\alpha }\]
Comparing it with eq (1) we get,
\[\begin{align}
& \dfrac{8}{7}=\dfrac{\alpha }{360-\alpha } \\
& \Rightarrow 7\alpha =8\times 360-8\alpha \\
& \Rightarrow 15\alpha =2880 \\
& \Rightarrow \alpha =192 \\
\end{align}\]
Thus, the angle came out to be 192 degrees. Hence, the correct option is (C).
Note: We have used ideal gas law here because it was mentioned in the question that the gas is ideal. Otherwise we would have to use real gas laws. Also while finding volume we have taken the ratio of angles as the radii for both are the same.
Complete step by step answer:
Pressure exerted by gas A= pressure exerted by gas B
Using ideal gas law, PV=nRT
\[P=\dfrac{nRT}{V}\]
So \[{{P}_{1}}={{P}_{2}}\]
\[\dfrac{{{n}_{1}}RT}{{{V}_{1}}}=\dfrac{{{n}_{2}}RT}{{{V}_{2}}}\]here temperature of the both of them are same and R is a gas constant.
Let us find out the number of moles, it is given by the formula. \[n=\dfrac{m}{M}\]
Here m is the given mass and M is the molecular mass of the gas. It is given that the given mass for both of the gas are the same.
\[\dfrac{{{n}_{1}}}{{{V}_{1}}}=\dfrac{{{n}_{2}}}{{{V}_{2}}}\]
\[\Rightarrow \dfrac{32}{{{V}_{1}}}=\dfrac{28}{{{V}_{2}}}\]
\[\Rightarrow \dfrac{{{V}_{2}}}{{{V}_{1}}}=\dfrac{8}{7}\]------(1)
It is clear from the figure that total angle is \[{{360}^{0}}\] and gas 2 makes an angle \[\alpha \]. Since radius for both is same, thus,
\[\dfrac{{{V}_{2}}}{{{V}_{1}}}=\dfrac{\alpha }{360-\alpha }\]
Comparing it with eq (1) we get,
\[\begin{align}
& \dfrac{8}{7}=\dfrac{\alpha }{360-\alpha } \\
& \Rightarrow 7\alpha =8\times 360-8\alpha \\
& \Rightarrow 15\alpha =2880 \\
& \Rightarrow \alpha =192 \\
\end{align}\]
Thus, the angle came out to be 192 degrees. Hence, the correct option is (C).
Note: We have used ideal gas law here because it was mentioned in the question that the gas is ideal. Otherwise we would have to use real gas laws. Also while finding volume we have taken the ratio of angles as the radii for both are the same.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




