
A refrigerator works on the principle of ______________ law of thermodynamics.
(a)- Zeroth
(b)- First
(c)- Second
(d)- Third
Answer
589.5k+ views
Hint: The law of thermodynamic says that the energy is transferred from a higher temperature to a lower temperature. But the energy has to be transferred from lower temperature to the higher temperature external work has to be applied.
Complete step by step answer:
According to the second law of thermodynamics: "It is impossible for a self-acting machine unless energy is provided by any external agency, to transfer heat from a body having lower temperature to another at a higher temperature". This is the basic principle used for the working of a refrigerator.
Let us understand the working of a refrigerator. Suppose in a refrigerator, the working substance absorbs a ${{Q}_{2}}$amount of heat from the cold reservoir at temperature${{T}_{2}}$. And a W amount of work is done on it by some external agency (a compressor pump driven by an electric motor) and rejects a larger amount of heat ${{Q}_{1}}$to the source at temperature${{T}_{1}}$. The diagram of working of the refrigerator is given below:
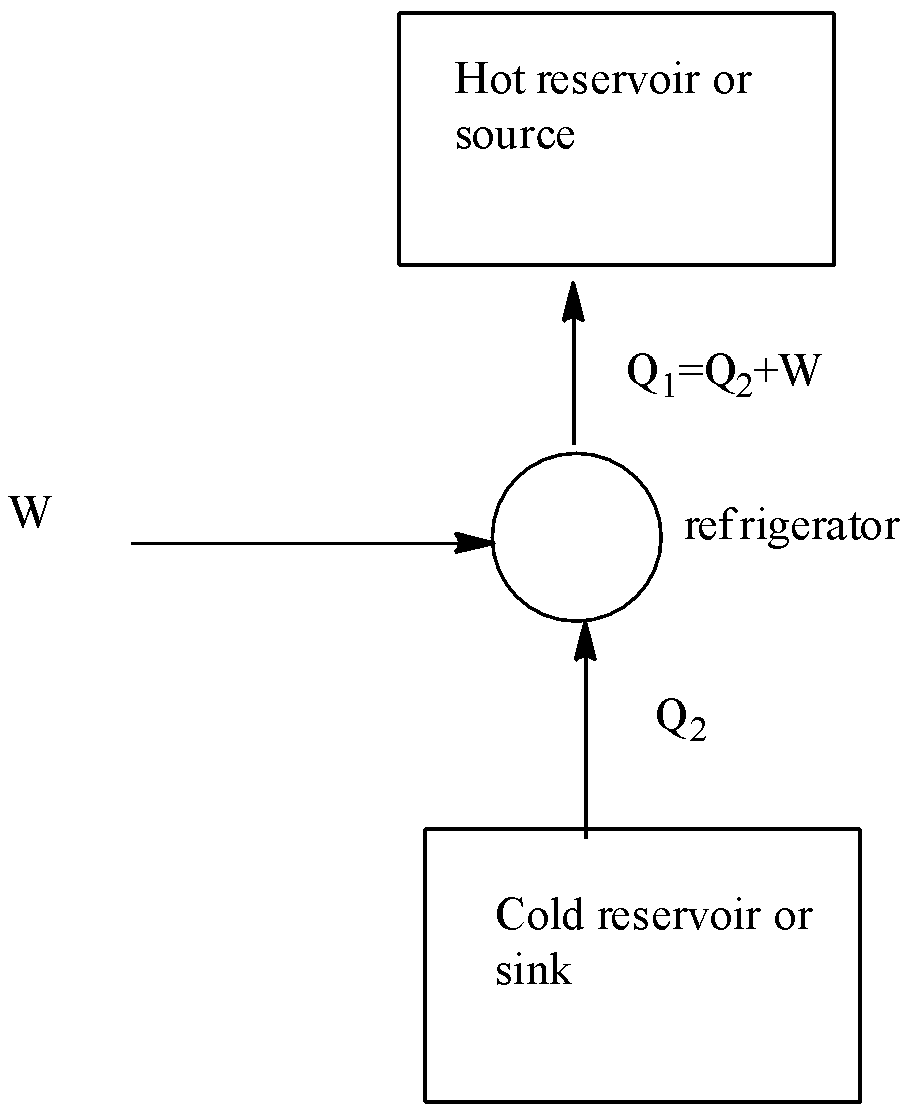
In the refrigerator used in our house, food and ice are the cold reservoir and the surrounding act as a hot reservoir. The work is done by electric motor and freons ($CC{{l}_{2}}{{F}_{2}}$)
So, this functioning is explained by the second law of thermodynamics.
So, the correct answer is an option (c)- Second.
Note: We can find the coefficient of performance of the refrigerator and it is the ratio of the amount of heat removed to the mechanical work done by the motor on the refrigerator. The formula is: $\beta =\frac{{{Q}_{2}}}{W}$ .
Complete step by step answer:
According to the second law of thermodynamics: "It is impossible for a self-acting machine unless energy is provided by any external agency, to transfer heat from a body having lower temperature to another at a higher temperature". This is the basic principle used for the working of a refrigerator.
Let us understand the working of a refrigerator. Suppose in a refrigerator, the working substance absorbs a ${{Q}_{2}}$amount of heat from the cold reservoir at temperature${{T}_{2}}$. And a W amount of work is done on it by some external agency (a compressor pump driven by an electric motor) and rejects a larger amount of heat ${{Q}_{1}}$to the source at temperature${{T}_{1}}$. The diagram of working of the refrigerator is given below:

In the refrigerator used in our house, food and ice are the cold reservoir and the surrounding act as a hot reservoir. The work is done by electric motor and freons ($CC{{l}_{2}}{{F}_{2}}$)
So, this functioning is explained by the second law of thermodynamics.
So, the correct answer is an option (c)- Second.
Note: We can find the coefficient of performance of the refrigerator and it is the ratio of the amount of heat removed to the mechanical work done by the motor on the refrigerator. The formula is: $\beta =\frac{{{Q}_{2}}}{W}$ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




