
A plot of the kinetic energy $\dfrac{1}{2}m{v^2}$ of ejected electrons as a function of the frequency (v) of incident radiation for four alkali metals \[\left( {{M_1},{M_2},{M_3},{M_4}} \right)\] is given below:
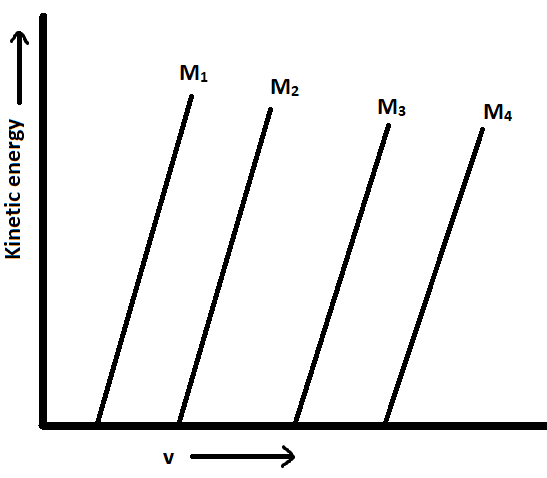
These alkali metals \[\left( {{M_1},{M_2},{M_3},{M_4}} \right)\] are respectively:
A) Li, Na, K and Rb
B) Rb, K, Na and Li
C) Na, K Li, and Rb
D) Rb, Li, Na and K

Answer
580.2k+ views
Hint: From the graph we can observe the value of work function because more work function decreases the size of the atom. With this a property among given alkali metals can be identified and then compared to the options to get the required values
Complete step by step answer:
The intercepts (value) on the frequency axis denotes ${v_0}$ and:
${v_0} = \dfrac{\phi }{h}$ where
$\phi $ is a work function.
More the value of ${v_0}$, more will be the value of work function.
From the graph we can observe that,
value of ${v_0}$ and also $\phi $ increases in the order:
\[{M_1} < {M_2} < {M_3} < {M_4}\]
Now it can be concluded,
${M_4}$ has maximum work function, shows that the electrons are tightly bound to the nucleus and thus the size is smaller.
${M_1}$ has minimum work function, shows that the electrons are loosely bound to the nucleus and thus the size is larger.
The given elements belong to the same group:
Li
Na
K
Rb
According to periodic property, when we move down the table, the atomic size increases
$ \Rightarrow $ The respective values will be:
${M_1}$ = Li
${M_2}$ = Na
${M_3}$ = K
${M_4}$ = Rb
Thus the correct option is (B).
Note:The atoms with strong force attraction are smaller because the nucleus attracts the valence electrons strongly towards itself shrinking and hence decreasing the size.
When we move downwards in a group, the number of valence shells of electrons goes on increasing, resulting in the increase of the size.
These alkali metals easily lose electrons and make alkaline (basic) solutions when mixed with water and thus are called as alkali metals. These block to s – block of the periodic table
Complete step by step answer:
The intercepts (value) on the frequency axis denotes ${v_0}$ and:
${v_0} = \dfrac{\phi }{h}$ where
$\phi $ is a work function.
More the value of ${v_0}$, more will be the value of work function.

From the graph we can observe that,
value of ${v_0}$ and also $\phi $ increases in the order:
\[{M_1} < {M_2} < {M_3} < {M_4}\]
Now it can be concluded,
${M_4}$ has maximum work function, shows that the electrons are tightly bound to the nucleus and thus the size is smaller.
${M_1}$ has minimum work function, shows that the electrons are loosely bound to the nucleus and thus the size is larger.
The given elements belong to the same group:
Li
Na
K
Rb
According to periodic property, when we move down the table, the atomic size increases
$ \Rightarrow $ The respective values will be:
${M_1}$ = Li
${M_2}$ = Na
${M_3}$ = K
${M_4}$ = Rb
Thus the correct option is (B).
Note:The atoms with strong force attraction are smaller because the nucleus attracts the valence electrons strongly towards itself shrinking and hence decreasing the size.
When we move downwards in a group, the number of valence shells of electrons goes on increasing, resulting in the increase of the size.
These alkali metals easily lose electrons and make alkaline (basic) solutions when mixed with water and thus are called as alkali metals. These block to s – block of the periodic table
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




