
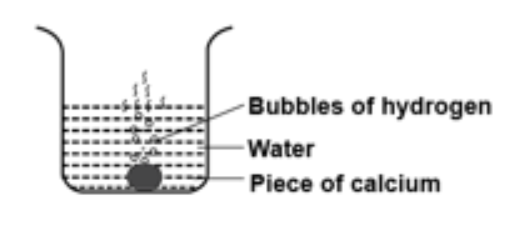
A piece of calcium metal is placed in water. A reaction takes place in which bubbles of hydrogen gas are seen. After the reaction stops, the solution is tested for its colour, temperature and action with litmus solution. Select the correct observation.
A.The solution is hot, milky white and turns red litmus blue
B.The solution is hot, colourless and turns blue litmus red
C.The solution is cold, milky and turns blue litmus red
D.The solution is cold, colourless and does not change the colour of litmus

Answer
586.2k+ views
Hint: Calcium is a whitish – silver coloured metal, which belongs to a group on the periodic table, also known as alkaline earth metals. Calcium exists in the form of various stable compounds. But once isolated as just elemental Calcium, it is highly reactive. Especially when it comes to water, calcium reacts vigorously due to its highly reactive nature.
Complete step by step answer:
-Before we move forward with the solution of this question, let us understand a few basic important concepts.
-The reaction of calcium with water results in the formation of 2 products, viz. calcium hydroxide and hydrogen gas. The chemical equation for the reaction between calcium and water is given by:
\[C{a_{(s)}} + {H_2}{O_{(l)}} \to Ca{(OH)_{2(aq)}} + {H_{2(g)}}\]
-The experimental setup that has been given to us states that a piece of calcium metal is placed in water. A reaction takes place in which bubbles of hydrogen gas are seen. After the reaction stops, the solution is tested for its colour, temperature, and action with litmus solution.
-The experimental setup results in the formation of an aqueous solution of Calcium hydroxide. The formation of bonds between the calcium atom and the \[ - OH\] group releases some energy. This results in the heating of the solution. Also, the compound formed is basic in nature. Because of this, it would turn a red litmus paper blue. When this solution reacts with the atmospheric carbon dioxide, the solution turns milky. This gives it the milky white colour.
-Hence, from the above data, we can conclude that the solution is hot, milky white and turns red litmus blue
Hence, Option A is the correct option.
Note:
Calcium hydroxide is an odourless white powder. It is used in industrial settings, such as sewage treatment, paper production, construction, and food processing. It also has medical and dental uses.
Complete step by step answer:
-Before we move forward with the solution of this question, let us understand a few basic important concepts.
-The reaction of calcium with water results in the formation of 2 products, viz. calcium hydroxide and hydrogen gas. The chemical equation for the reaction between calcium and water is given by:
\[C{a_{(s)}} + {H_2}{O_{(l)}} \to Ca{(OH)_{2(aq)}} + {H_{2(g)}}\]
-The experimental setup that has been given to us states that a piece of calcium metal is placed in water. A reaction takes place in which bubbles of hydrogen gas are seen. After the reaction stops, the solution is tested for its colour, temperature, and action with litmus solution.
-The experimental setup results in the formation of an aqueous solution of Calcium hydroxide. The formation of bonds between the calcium atom and the \[ - OH\] group releases some energy. This results in the heating of the solution. Also, the compound formed is basic in nature. Because of this, it would turn a red litmus paper blue. When this solution reacts with the atmospheric carbon dioxide, the solution turns milky. This gives it the milky white colour.
-Hence, from the above data, we can conclude that the solution is hot, milky white and turns red litmus blue
Hence, Option A is the correct option.
Note:
Calcium hydroxide is an odourless white powder. It is used in industrial settings, such as sewage treatment, paper production, construction, and food processing. It also has medical and dental uses.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Which Country is Called "The Land of Festivals"?

What type of cell is found in the Seminiferous tub class 10 biology CBSE

What are the public facilities provided by the government? Also explain each facility




