
A mixture of gases $ {O_2} $ , $ {H_2} $ and $ CO $ are taken in a closed vessel containing charcoal. The graph that represents the correct behaviour of pressure with time is:
A.
B.
C.
D.




Answer
520.8k+ views
Hint :Adsorption: It is the tendency of a solid substance to attract molecules of gases and liquids to fill the pores or sites available at its surface. Adsorption is further categorized into two parts i.e., physical adsorption and chemical adsorption.
Complete Step By Step Answer:
Activated carbon also known as charcoal is an excellent adsorbent because it is a highly spongy material and gives a very large surface area to which external molecules may adsorb. Its major application is to remove impurities from a stream of air by physically attaching contaminated particles to the surface of activated carbon.
Now, we know that the rate of adsorption of a gas is directly proportional to the pressure of the system i.e., if the pressure of the system is increased then an increase in the rate of adsorption also increases.
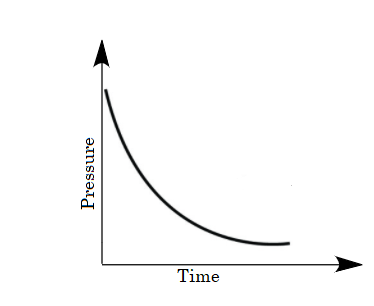
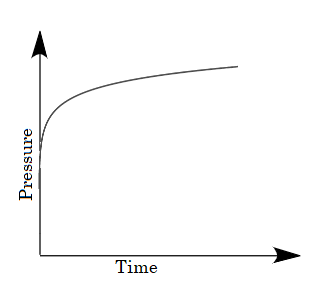
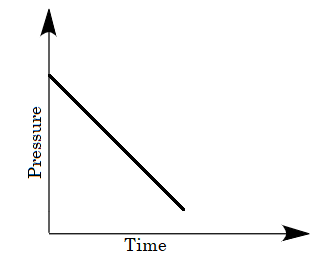
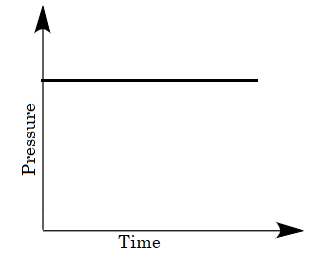
For the given case, a mixture of gases is taken in the closed vessel i.e., as the time increases the molecules of gases get adsorbed and the vacancies of pores on charcoal decreases due to which the rate of adsorption decreases with increase in time. Hence a decrease in pressure is also observed on increasing time. So, options (B) and (D) are eliminated because in the given graphs pressure is not decreasing with time.
The change in pressure with time will not be a linear decrease because the molecules of gases are not adsorbed linearly. The molecules of gases absorb as per the sites available per unit area. Therefore option (C) is eliminated as well.
As the decrease in pressure on increasing time is non-linear. Thus pressure varies with time as follows:
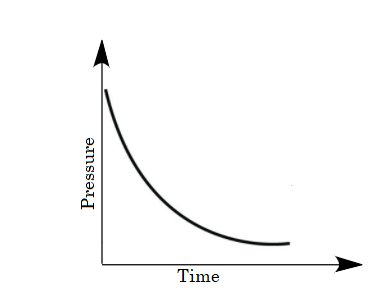
So, option (A) is the correct answer.
Note :
Do not get confused between the terms adsorption and absorption. The major difference is that absorption is a phenomenon in which the gas or liquid molecules are fully dissolved whereas in adsorption, the molecules are stuck at the surface of the solid substance.
Complete Step By Step Answer:
Activated carbon also known as charcoal is an excellent adsorbent because it is a highly spongy material and gives a very large surface area to which external molecules may adsorb. Its major application is to remove impurities from a stream of air by physically attaching contaminated particles to the surface of activated carbon.
Now, we know that the rate of adsorption of a gas is directly proportional to the pressure of the system i.e., if the pressure of the system is increased then an increase in the rate of adsorption also increases.
For the given case, a mixture of gases is taken in the closed vessel i.e., as the time increases the molecules of gases get adsorbed and the vacancies of pores on charcoal decreases due to which the rate of adsorption decreases with increase in time. Hence a decrease in pressure is also observed on increasing time. So, options (B) and (D) are eliminated because in the given graphs pressure is not decreasing with time.
The change in pressure with time will not be a linear decrease because the molecules of gases are not adsorbed linearly. The molecules of gases absorb as per the sites available per unit area. Therefore option (C) is eliminated as well.
As the decrease in pressure on increasing time is non-linear. Thus pressure varies with time as follows:

So, option (A) is the correct answer.
Note :
Do not get confused between the terms adsorption and absorption. The major difference is that absorption is a phenomenon in which the gas or liquid molecules are fully dissolved whereas in adsorption, the molecules are stuck at the surface of the solid substance.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




