
When a mixture of calcium acetate as calcium formate is heated, we get
(A) Acetone
(B) Acetic acid
(C) Acetaldehyde
(D) Methanol
Answer
571.8k+ views
Hint: In chemistry, every special group is aimed for easy identification. Two or more compounds bond to form another compound or another fictional group. The way by which a compound will react with another compound depends on the molecular structure of the compound. The arrangement of the functional groups in space will be responsible for the formation of the compound.
Complete step by step answer:
Calcium is the calcium compound of acetic acid. $(C{H_3}COOH)$ . When acetic acid reacts with a compound of calcium, calcium atom is retained by the compound thus forms the compound calcium acetate.
The structure of calcium acetate is as given in the figure below.
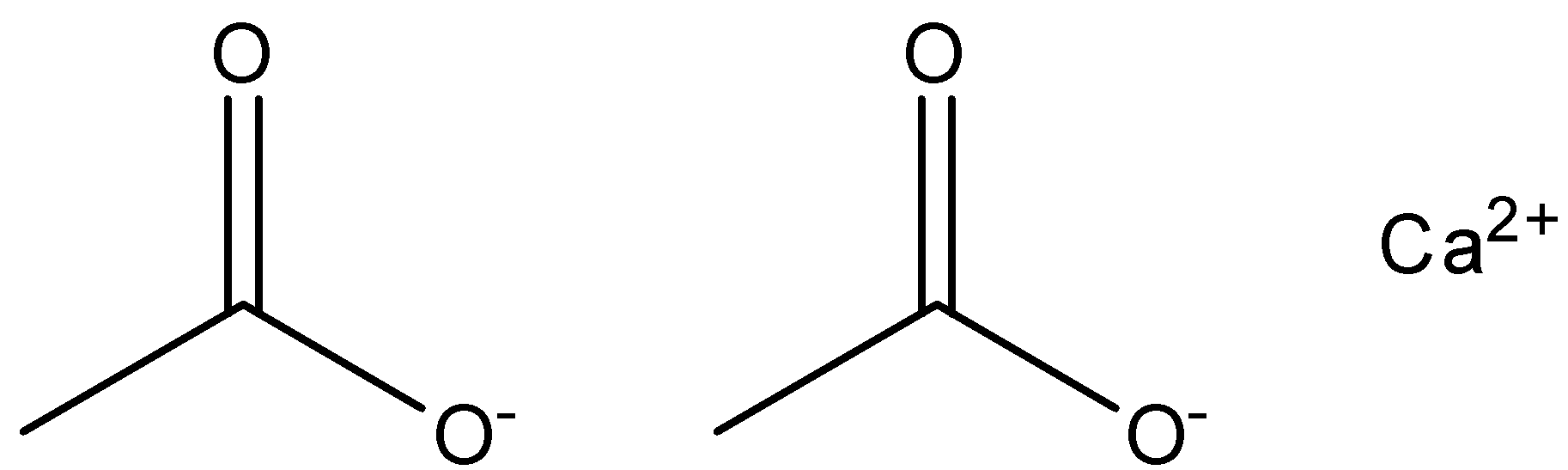
While calcium formate is the calcium compound of formic acid. Formic acid reacts and retains the calcium from another compound to form the compound of calcium formate. It has the formula of ${(HCOO)_2}Ca$ .
The structure of calcium formate is represented in the image below.
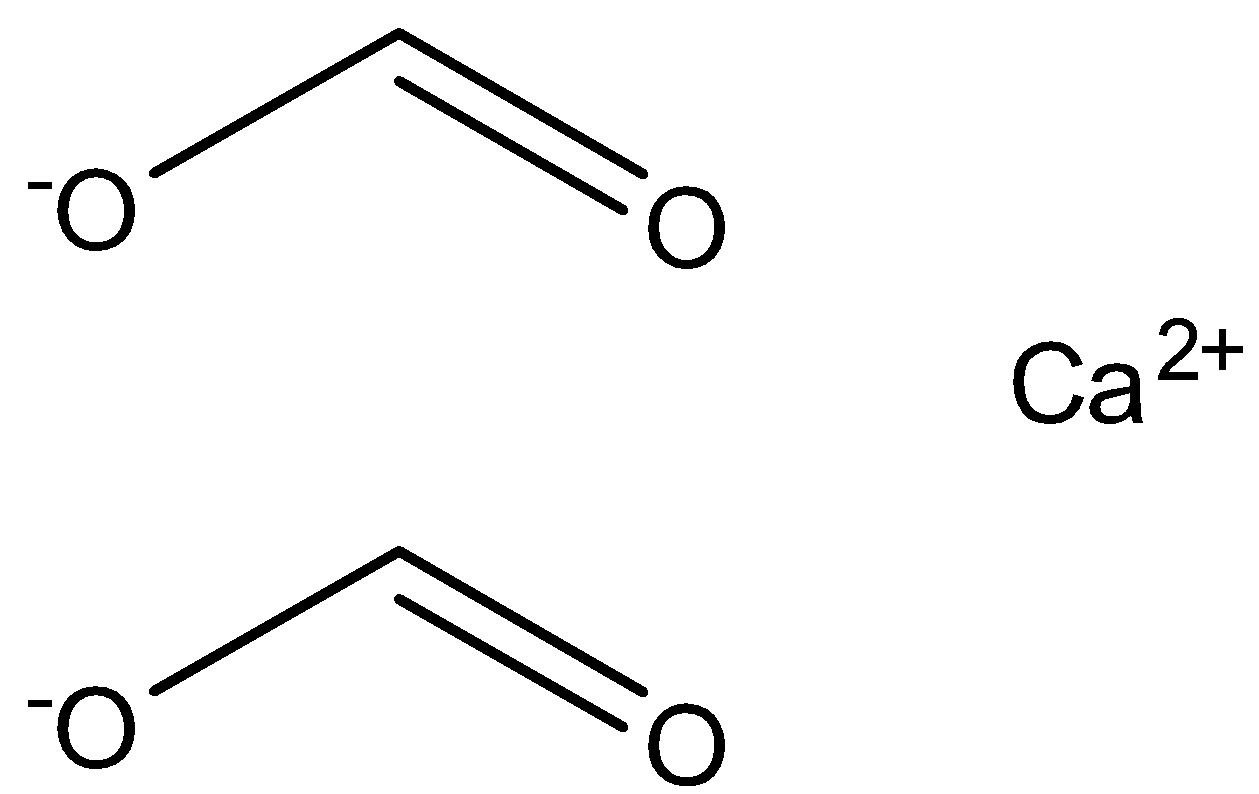
When calcium formate reacts with calcium acetate it leads to the formation of the compound acetaldehyde. The reaction depicting the above reaction is given below.
$Calcium{{ Acetate + Calcium Formate}}\xrightarrow[{}]{}2CaC{o_3} + Acetaldehyde$
Aldehydes are the parent functional group of acetaldehyde.
Acetaldehyde is a highly reactive and toxic compound but has large applications in the industrial domain.
So, the correct answer is Option C .
Note: Acetaldehyde is generally produced by the oxidation of ethylene by the reaction called the Wacker process. It is the process I which ethylene is oxidized in the presence of palladium
Acetaldehyde is found In coffee naturally
Complete step by step answer:
Calcium is the calcium compound of acetic acid. $(C{H_3}COOH)$ . When acetic acid reacts with a compound of calcium, calcium atom is retained by the compound thus forms the compound calcium acetate.
The structure of calcium acetate is as given in the figure below.

While calcium formate is the calcium compound of formic acid. Formic acid reacts and retains the calcium from another compound to form the compound of calcium formate. It has the formula of ${(HCOO)_2}Ca$ .
The structure of calcium formate is represented in the image below.

When calcium formate reacts with calcium acetate it leads to the formation of the compound acetaldehyde. The reaction depicting the above reaction is given below.
$Calcium{{ Acetate + Calcium Formate}}\xrightarrow[{}]{}2CaC{o_3} + Acetaldehyde$
Aldehydes are the parent functional group of acetaldehyde.
Acetaldehyde is a highly reactive and toxic compound but has large applications in the industrial domain.
So, the correct answer is Option C .
Note: Acetaldehyde is generally produced by the oxidation of ethylene by the reaction called the Wacker process. It is the process I which ethylene is oxidized in the presence of palladium
Acetaldehyde is found In coffee naturally
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




