
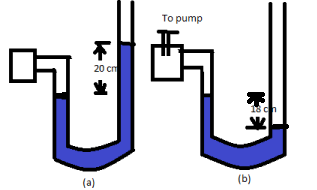
A manometer reads the pressure of a gas in an enclosure as shown in fig (a). When a pump removes some of the gas, the manometer reads as in fig (b). The liquid used in the manometers is mercury and the atmospheric pressure is $76\,cm$ of mercury.
a. Give the absolute and gauge pressure of the gas in the enclosure for cases (a) and (b), in units of $cm$ of mercury.
b. How would the levels change in case (b) if $13.6\,cm$ of water (immiscible with mercury) are poured into the right limb of the manometer? (Ignore the small change in the volume of the gas.

Answer
579k+ views
Hint:
Here for case (1) in figure (a) and figure (b) we have to calculate the absolute pressure using the formula of absolute pressure.
For case (2) we have to first calculate the pressure for the right and left limb and then equate them to find the height.
Complete step by step solution:
Given,
Atmospheric pressure ${P_ \circ } = 76\,cm\,\,{\text{of}}\,{\text{Hg}}$
Case (1)
For figure (a)
Gauge pressure is given by the gap between the mercury levels in the two limbs.
So, Gauge pressure $ = 20\,cm\,\,{\text{of}}\,{\text{Hg}}$
We know that,
Absolute pressure = atmospheric pressure + gauge pressure
$ = 76\,cm + 20\,cm = 96\,cm\,\,{\text{of}}\,{\text{Hg}}$
For figure (b)
Difference between mercury amounts in the two limbs $ = - 18\,cm$
So, gauge pressure $ = - 18\,cm\,\,{\text{of}}\,{\text{Hg}}$
Absolute pressure = atmospheric pressure + gauge pressure
$ = 76\,cm - 18\,cm = 58\,cm$
Case (2)
Given, $13.6\,cm$ of water (immiscible with mercury) is poured into the right limb of the manometer.
So, relative density of mercury $ = 13.6\,cm$
Therefore, $1\,cm$ of mercury is equal to a column of $13.6\,cm$ of water.
Now, let the difference between the mercury levels in the two limbs be $h$
So, the right limb pressure is given by-
Pressure = atmospheric pressure + $1\,cm\,\,{\text{of}}\,{\text{Hg}}$
$ = 76 + 1 = 77\,cm\,\,{\text{of}}\,{\text{Hg}}$ ...... (i)
In the left limb, the mercury column will rise.
Hence, pressure in the left limb-
Pressure= atmospheric pressure + $1\,cm\,\,{\text{of}}\,{\text{Hg}}$
$ = 58 + h$ ...... (ii)
From equations (i) and (ii), we get-
$
77 = 58 + h \\
h = 19\,cm \\
$
Hence, the levels change in case (b) if $13.6\,cm$ of water (immiscible with mercury) are poured into the right limb of the manometer by $19\,cm$.
Note:
Here we have to be careful for the gauge pressure in case of figure (b), as the gauge pressure here is of negative value since the level of mercury is less in the right limb than in the left limb.
Here for case (1) in figure (a) and figure (b) we have to calculate the absolute pressure using the formula of absolute pressure.
For case (2) we have to first calculate the pressure for the right and left limb and then equate them to find the height.
Complete step by step solution:
Given,
Atmospheric pressure ${P_ \circ } = 76\,cm\,\,{\text{of}}\,{\text{Hg}}$
Case (1)
For figure (a)
Gauge pressure is given by the gap between the mercury levels in the two limbs.
So, Gauge pressure $ = 20\,cm\,\,{\text{of}}\,{\text{Hg}}$
We know that,
Absolute pressure = atmospheric pressure + gauge pressure
$ = 76\,cm + 20\,cm = 96\,cm\,\,{\text{of}}\,{\text{Hg}}$
For figure (b)
Difference between mercury amounts in the two limbs $ = - 18\,cm$
So, gauge pressure $ = - 18\,cm\,\,{\text{of}}\,{\text{Hg}}$
Absolute pressure = atmospheric pressure + gauge pressure
$ = 76\,cm - 18\,cm = 58\,cm$
Case (2)
Given, $13.6\,cm$ of water (immiscible with mercury) is poured into the right limb of the manometer.
So, relative density of mercury $ = 13.6\,cm$
Therefore, $1\,cm$ of mercury is equal to a column of $13.6\,cm$ of water.
Now, let the difference between the mercury levels in the two limbs be $h$
So, the right limb pressure is given by-
Pressure = atmospheric pressure + $1\,cm\,\,{\text{of}}\,{\text{Hg}}$
$ = 76 + 1 = 77\,cm\,\,{\text{of}}\,{\text{Hg}}$ ...... (i)
In the left limb, the mercury column will rise.
Hence, pressure in the left limb-
Pressure= atmospheric pressure + $1\,cm\,\,{\text{of}}\,{\text{Hg}}$
$ = 58 + h$ ...... (ii)
From equations (i) and (ii), we get-
$
77 = 58 + h \\
h = 19\,cm \\
$
Hence, the levels change in case (b) if $13.6\,cm$ of water (immiscible with mercury) are poured into the right limb of the manometer by $19\,cm$.
Note:
Here we have to be careful for the gauge pressure in case of figure (b), as the gauge pressure here is of negative value since the level of mercury is less in the right limb than in the left limb.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




