
What is a major resonance contributor?
Answer
510.6k+ views
Hint: Resonance contributors are those structures which contribute to the resonance of the whole compound. A major resonance contributor is decided based on its energy, stability i.e., if its octet is fully filled or not.
Complete answer:
The order is as following
The most significant resonance contributor will be the one having maximum number of fully filled octets.
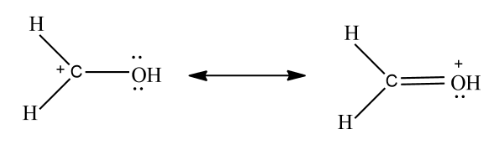
In the above example on the left Carbon has an incomplete octet while on the right all atoms have completely filled octet so the compound on the right is a more significant resonance contributor.
The most significant resonance contributor has the least number of formal changes associated with it.
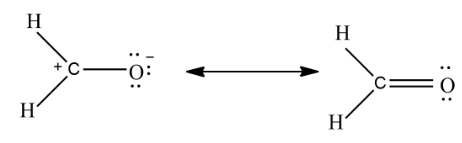
In the above example, the left compound is associated with two formal changes while the compound on the right is associated with no formal changes, so the compound on the right is a more significant resonance contributor.
The most significant resonance contributor has negative formal changes on most electronegative atoms and positive formal changes on least electronegative atoms
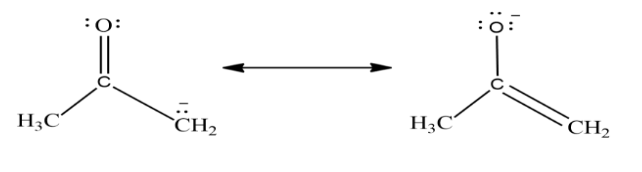
In the above example on the left carbon is associated with negative formal change while on the right oxygen is associated with negative change as oxygen is more electronegative than carbon so compound on right is a more significant resonance contributor.
The most significant resonance contributor has the maximum number of covalent bonds.
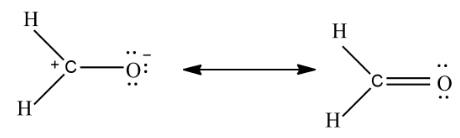
In the above example the compound on the left has three covalent bonds while the compound on right has four covalent bonds so the compound on right is a more significant resonance contributor.
Note:
There is an order to decide major resonance contributors. it has to follow this order to become a major resonance contributor. In order to decide the major resonance contributor order should be followed strictly as given otherwise it could be wrong.
Complete answer:
The order is as following
The most significant resonance contributor will be the one having maximum number of fully filled octets.

In the above example on the left Carbon has an incomplete octet while on the right all atoms have completely filled octet so the compound on the right is a more significant resonance contributor.
The most significant resonance contributor has the least number of formal changes associated with it.

In the above example, the left compound is associated with two formal changes while the compound on the right is associated with no formal changes, so the compound on the right is a more significant resonance contributor.
The most significant resonance contributor has negative formal changes on most electronegative atoms and positive formal changes on least electronegative atoms

In the above example on the left carbon is associated with negative formal change while on the right oxygen is associated with negative change as oxygen is more electronegative than carbon so compound on right is a more significant resonance contributor.
The most significant resonance contributor has the maximum number of covalent bonds.

In the above example the compound on the left has three covalent bonds while the compound on right has four covalent bonds so the compound on right is a more significant resonance contributor.
Note:
There is an order to decide major resonance contributors. it has to follow this order to become a major resonance contributor. In order to decide the major resonance contributor order should be followed strictly as given otherwise it could be wrong.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




