
(a) List three properties of ionic compounds.
(b) Show the formation of \[NaCl\] by transfer of electron
(c) How is it that ionic compounds in the solid state do not conduct electricity but they do so when in molten state?
Answer
525.1k+ views
Hint: We must know the concepts of ionic compounds, their formation, characteristics are important to remember. Ionic compounds are compounds composed of ions, charged particles that form when an atom (or group of atoms) gains or loses electrons. (A cation is a positively charged ion; an anion is a negatively charged ion.)
Complete step by step solution:
(a) Properties of ionic compounds:
1. The ionic compounds are solids at room temperature. (For example $NaCl{\text{ }}and{\text{ }}LiBr$)
They are formed by gaining or losing electrons. If an element loses its electrons then it carries positive charge or if an element gain electrons then it carries negative charge
2. Ionic compounds usually soluble in water
They shows high melting and boiling point due to oppositely charged partials with strong electrostatic force of attraction
3. They are good conductors of electricity
They are easily soluble in water and give ions. So, the free ions help to conduct electricity.
(b) Presentation of \[NaCl\] formation by transfer of electron
The atomic number of sodium(Na) is 11 so electrons present in the last shell are 1 whereas the atomic number of chlorine(Cl) is 17 so electrons present in the last shell is 7. So Sodium transfers or gives its 1 electron to chlorine and become octet stabilized.chem This is complete transfer of electrons so \[NaCl\] is ionic compound
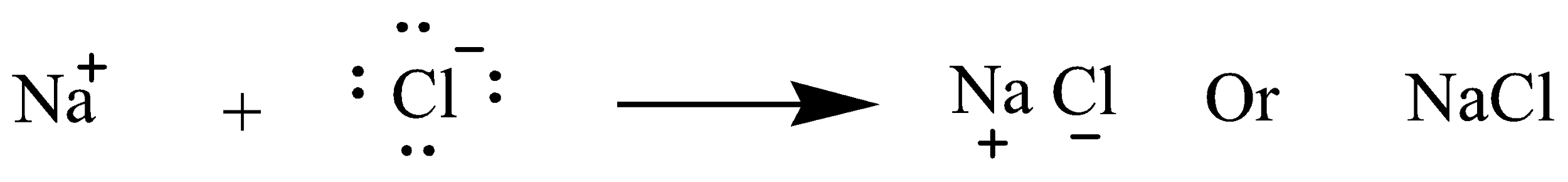
(c ) In solid state, the ions in the compounds are not free to move and are held in crystal lattices, so the ions are unable to carry electricity. To conduct electricity, ions need to be free and moving. In molten state, they become mobile and thus are able to conduct electricity
Note: Different types of compounds are present, and the characteristics of compounds depend upon their type of bonds that they form. Ionic compounds are formed from ionic bonds, and so their properties are with respect to ionic bonds.
Complete step by step solution:
(a) Properties of ionic compounds:
1. The ionic compounds are solids at room temperature. (For example $NaCl{\text{ }}and{\text{ }}LiBr$)
They are formed by gaining or losing electrons. If an element loses its electrons then it carries positive charge or if an element gain electrons then it carries negative charge
2. Ionic compounds usually soluble in water
They shows high melting and boiling point due to oppositely charged partials with strong electrostatic force of attraction
3. They are good conductors of electricity
They are easily soluble in water and give ions. So, the free ions help to conduct electricity.
(b) Presentation of \[NaCl\] formation by transfer of electron
The atomic number of sodium(Na) is 11 so electrons present in the last shell are 1 whereas the atomic number of chlorine(Cl) is 17 so electrons present in the last shell is 7. So Sodium transfers or gives its 1 electron to chlorine and become octet stabilized.chem This is complete transfer of electrons so \[NaCl\] is ionic compound

(c ) In solid state, the ions in the compounds are not free to move and are held in crystal lattices, so the ions are unable to carry electricity. To conduct electricity, ions need to be free and moving. In molten state, they become mobile and thus are able to conduct electricity
Note: Different types of compounds are present, and the characteristics of compounds depend upon their type of bonds that they form. Ionic compounds are formed from ionic bonds, and so their properties are with respect to ionic bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




