
A liquid is contained in a vessel. The liquid-solid adhesive force is very weak as compared to the cohesive force in the liquid. The shape of the liquid surface will be
A. Horizontal
B. Vertical
C. Concave
D. Convex
Answer
579.6k+ views
Hint: Cohesive force is the force of attraction between the liquid molecules. If the cohesive force is greater than the adhesive force between liquid molecules and the molecules of the vessel, then the angle of contact made by the liquid will be greater than ${90^ \circ }$. That is the surface will curve outward.
Complete step by step answer:
The shape of a liquid surface in a container will depend upon the cohesive and adhesive forces. The cohesive force is the force of attraction between the molecules of the liquid or we can say it is the force of attraction between similar molecules. Whereas the adhesive force is the force of attraction between the molecules of the liquid with the molecules of the container. That is adhesive force is the force of attraction between dissimilar molecules. If the cohesive force is greater then, the liquid molecules will tend to stay together. The surface tension of the solid-liquid interface is then greater than the surface tension of the liquid and air interface. So, they will not wet the surface of the container.
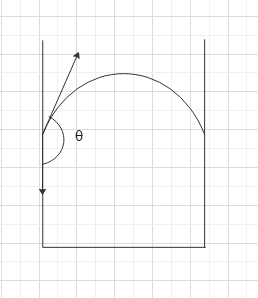
The angle of contact $\theta $ made by the tangent to the surface of the liquid at the point of contact of liquid with the vessel to the vertical surface of the vessel inside the liquid in such cases will be an obtuse angle. That is more than $90^\circ $. So, the liquid will curve outward in a convex shape. For example, in the case of mercury in a glass tube, the surface of the mercury will be in a convex shape since the cohesive force between the mercury molecules is greater than the force of attraction between the glass molecules and the mercury.
Hence, the correct answer is option D.
Note:
In the case where the cohesive force is less than the adhesive force, then the liquid will wet the surface of the container resulting in the contact angle to be less than $90^\circ $. Then, the shape of the surface will then be concave.
Complete step by step answer:
The shape of a liquid surface in a container will depend upon the cohesive and adhesive forces. The cohesive force is the force of attraction between the molecules of the liquid or we can say it is the force of attraction between similar molecules. Whereas the adhesive force is the force of attraction between the molecules of the liquid with the molecules of the container. That is adhesive force is the force of attraction between dissimilar molecules. If the cohesive force is greater then, the liquid molecules will tend to stay together. The surface tension of the solid-liquid interface is then greater than the surface tension of the liquid and air interface. So, they will not wet the surface of the container.

The angle of contact $\theta $ made by the tangent to the surface of the liquid at the point of contact of liquid with the vessel to the vertical surface of the vessel inside the liquid in such cases will be an obtuse angle. That is more than $90^\circ $. So, the liquid will curve outward in a convex shape. For example, in the case of mercury in a glass tube, the surface of the mercury will be in a convex shape since the cohesive force between the mercury molecules is greater than the force of attraction between the glass molecules and the mercury.
Hence, the correct answer is option D.
Note:
In the case where the cohesive force is less than the adhesive force, then the liquid will wet the surface of the container resulting in the contact angle to be less than $90^\circ $. Then, the shape of the surface will then be concave.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




