
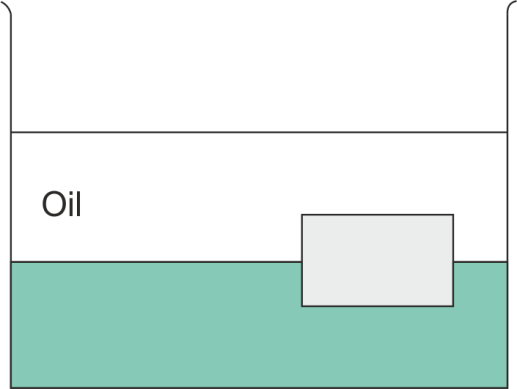
A layer of oil with density $724\text{ kg/}{{\text{m}}^{3}}$ floats on water of density $1000\text{ kg/}{{\text{m}}^{3}}$. A block floats at the oil water interface with $\dfrac{1}{6}$ of its volume in oil and $\dfrac{5}{6}$ of its volume in water as shown in the figure. What is the density of the block?
(A) $1024\text{ kg/}{{\text{m}}^{3}}$
(B) $1276\text{ kg/}{{\text{m}}^{3}}$
(C) $776\text{ kg/}{{\text{m}}^{3}}$
(D) $951\text{ kg/}{{\text{m}}^{3}}$

Answer
550.5k+ views
Hint: Oil molecules are only attracted to other oil molecules. Water is more dense than oil; they can’t mix oil floats above the water. Density is the weight of a substance for a specific volume.
Density$=\dfrac{mass}{volume}$, $volume\times density=mass$
Formula used:
By using Archimedes Principle
Upthrust $=$ Weight of the liquid displaced.
$\left( {{V}_{Pb}} \right)g=\left( {{V}_{o{{\rho }_{1}}}} \right)g+\left( {{V}_{W{{\rho }_{2}}}} \right)g$
$\left( {{V}_{Pb}} \right)g\to $Volume of block $\times $density of block $\times $ specific gravity.
$g\to $specific gravity
${{V}_{0}}\to $ Volume of oil.
${{\rho }_{1}}\to $ Density of oil
${{V}_{w}}\to $ Volume of water
${{\rho }_{2}}\to $ Density of water
Complete solution:
Given data:
Density of oil ${{\rho }_{1}}=724\text{ kg/}{{\text{m}}^{3}}$
Density of water ${{\rho }_{2}}=1000\text{ kg/}{{\text{m}}^{3}}$
According to Archimedes principle
Upthrust $=$ Weight of liquid displaced
${{\left( {{V}_{\rho }} \right)}_{b}}g={{V}_{0}}{{\rho }_{1}}g+{{V}_{w}}{{\rho }_{2}}g$
$\Rightarrow {{\left( {{V}_{\rho }} \right)}_{b}}g=\dfrac{V}{6}\left( 724 \right)g+\dfrac{5V}{6}\left( 1000 \right)g$
Where, ${{V}_{b}}\to $ block volume, ${{\rho }_{b}}\to $ density of block
${{V}_{0}}=\dfrac{V}{6},{{V}_{\infty }}=\dfrac{5V}{6}$
$\Rightarrow {{\left( {{V}_{\rho }} \right)}_{b}}g=\left( \dfrac{724V}{6}+\dfrac{5000V}{6} \right)g$
${{\left( V\rho \right)}_{b}}=\dfrac{724V}{6}+\dfrac{5000V}{6}$
$V{{\rho }_{b}}=\dfrac{5724}{6}V$
${{\rho }_{b}}=\dfrac{5724}{6}$
${{\rho }_{b}}=954\text{ kg/}{{\text{m}}^{3}}$
Therefore the density of the block is $954\text{ kg/}{{\text{m}}^{3}}$ i.e. option (d) is correct.
Additional information:
Density of liquid water is $1\text{ g/c}{{\text{m}}^{3}}$ and vegetable oil is $0.93\text{ g/c}{{\text{m}}^{3}}$. Ice has a density of $0.92\text{ g/c}{{\text{m}}^{3}}$ Which is why it floats on water and oil.
Note:
Oil is less dense than water. The molecules that make the oil are larger than those that make up water, so they cannot pick as tightly together as the water molecules.
Density$=\dfrac{mass}{volume}$, $volume\times density=mass$
Formula used:
By using Archimedes Principle
Upthrust $=$ Weight of the liquid displaced.
$\left( {{V}_{Pb}} \right)g=\left( {{V}_{o{{\rho }_{1}}}} \right)g+\left( {{V}_{W{{\rho }_{2}}}} \right)g$
$\left( {{V}_{Pb}} \right)g\to $Volume of block $\times $density of block $\times $ specific gravity.
$g\to $specific gravity
${{V}_{0}}\to $ Volume of oil.
${{\rho }_{1}}\to $ Density of oil
${{V}_{w}}\to $ Volume of water
${{\rho }_{2}}\to $ Density of water
Complete solution:
Given data:
Density of oil ${{\rho }_{1}}=724\text{ kg/}{{\text{m}}^{3}}$
Density of water ${{\rho }_{2}}=1000\text{ kg/}{{\text{m}}^{3}}$
According to Archimedes principle
Upthrust $=$ Weight of liquid displaced
${{\left( {{V}_{\rho }} \right)}_{b}}g={{V}_{0}}{{\rho }_{1}}g+{{V}_{w}}{{\rho }_{2}}g$
$\Rightarrow {{\left( {{V}_{\rho }} \right)}_{b}}g=\dfrac{V}{6}\left( 724 \right)g+\dfrac{5V}{6}\left( 1000 \right)g$
Where, ${{V}_{b}}\to $ block volume, ${{\rho }_{b}}\to $ density of block
${{V}_{0}}=\dfrac{V}{6},{{V}_{\infty }}=\dfrac{5V}{6}$
$\Rightarrow {{\left( {{V}_{\rho }} \right)}_{b}}g=\left( \dfrac{724V}{6}+\dfrac{5000V}{6} \right)g$
${{\left( V\rho \right)}_{b}}=\dfrac{724V}{6}+\dfrac{5000V}{6}$
$V{{\rho }_{b}}=\dfrac{5724}{6}V$
${{\rho }_{b}}=\dfrac{5724}{6}$
${{\rho }_{b}}=954\text{ kg/}{{\text{m}}^{3}}$
Therefore the density of the block is $954\text{ kg/}{{\text{m}}^{3}}$ i.e. option (d) is correct.
Additional information:
Density of liquid water is $1\text{ g/c}{{\text{m}}^{3}}$ and vegetable oil is $0.93\text{ g/c}{{\text{m}}^{3}}$. Ice has a density of $0.92\text{ g/c}{{\text{m}}^{3}}$ Which is why it floats on water and oil.
Note:
Oil is less dense than water. The molecules that make the oil are larger than those that make up water, so they cannot pick as tightly together as the water molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




