
What is A in reaction below?
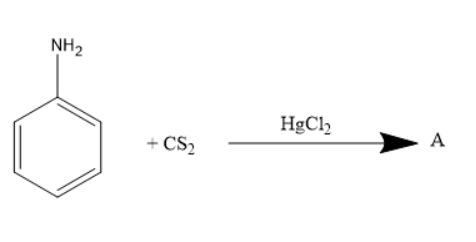
What is A?
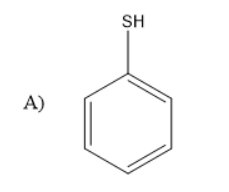
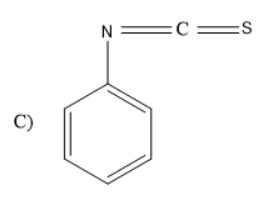
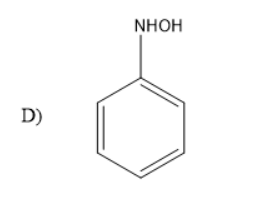





Answer
504.3k+ views
Hint: Aromatic compounds are the compounds that are cyclic, planar, conjugation of pi-electrons and obeying Huckel’s rule. Aniline is an aromatic compound and undergoes substitution reactions. Aniline is treated with carbon disulphide in presence of mercury salts to form phenyl isothiocyanate.
Complete answer:
Given compound is aniline. Aniline is an aromatic compound as it is cyclic, planar i.e., all the carbon atoms are \[s{p^2}\], conjugation of \[\pi \] electrons and the compound obey Huckel’s rule. The huckel’s rule is given by \[\left( {4n + 2} \right)\pi \] electrons, where n is a whole number.
As the aniline consists of \[6\pi \]electrons satisfying Huckel's condition. It is an aromatic compound.
When aniline is treated with carbon disulphide in presence of mercury salts like \[HgC{l_2}\], the amine group in the aniline rearranges to form iso thiocyanate group. Thus, phenyl isothiocyanate will be formed.
This reaction can be one of the important named reactions in organic chemistry called the Hoffmann mustard oil reaction.
Thus, the given reaction is Hoffmann mustard oil reaction in which the formed products are isothiocyanates which have very unpleasant or pungent odour and are very dangerous as they consist of cyanides or nitriles. Thus, the product formed will be
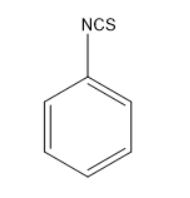
So, the correct answer is “Option C”.
Note:
Cyanides have the functional group as -CN, but the thio cyanate indicates the -NC, the iso thio cyanates have the functional group as -NCS. The iso thio cyanates have a very pungent odour and not be inhaled. So, precautions must be taken while dealing with this reaction.
Complete answer:
Given compound is aniline. Aniline is an aromatic compound as it is cyclic, planar i.e., all the carbon atoms are \[s{p^2}\], conjugation of \[\pi \] electrons and the compound obey Huckel’s rule. The huckel’s rule is given by \[\left( {4n + 2} \right)\pi \] electrons, where n is a whole number.
As the aniline consists of \[6\pi \]electrons satisfying Huckel's condition. It is an aromatic compound.
When aniline is treated with carbon disulphide in presence of mercury salts like \[HgC{l_2}\], the amine group in the aniline rearranges to form iso thiocyanate group. Thus, phenyl isothiocyanate will be formed.
This reaction can be one of the important named reactions in organic chemistry called the Hoffmann mustard oil reaction.
Thus, the given reaction is Hoffmann mustard oil reaction in which the formed products are isothiocyanates which have very unpleasant or pungent odour and are very dangerous as they consist of cyanides or nitriles. Thus, the product formed will be

So, the correct answer is “Option C”.
Note:
Cyanides have the functional group as -CN, but the thio cyanate indicates the -NC, the iso thio cyanates have the functional group as -NCS. The iso thio cyanates have a very pungent odour and not be inhaled. So, precautions must be taken while dealing with this reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




