
A hydrocarbon A (V.D = 36) forms only one monochloro substitution product. A will be
(A) isopentane
(C) cyclohexane
(D) methyl cyclohexane
Answer
569.4k+ views
Hint: We can use the vapour density given to find the hydrocarbon A. Twice of the vapour density of the hydrocarbon A will give the molecular weight of the compound. On knowing the molecular mass, we can easily find the hydrocarbon A.
Complete Solution :
Vapour density of the hydrocarbon is given which is 36.
Molecular weight of the hydrocarbon \[ = 2 \times 36 = 72\]
Calculation of the molecular weight suggests that it is alkane.
The general formula of alkane is \[{C_n}{H_{2n + 2}}\].
Hydrocarbon A \[ = {C_n}{H_{2n + 2}} = 12n + 2n + 2 = 72\]
The hydrocarbon formed is \[{C_5}{H_{12}}\].
The hydrocarbon A on chlorination will give monochloro substituted product i.e. 1-Chloro-2,2-dimethylpropane.
The hydrocarbon A with the formula \[{C_5}{H_{12}}\] is Neopentane or 2,2-Dimethylpropane.
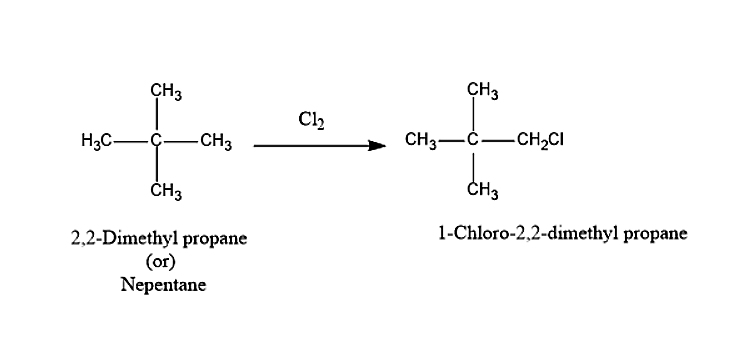
So, the correct answer is “Option B”. neo-pentane.
Additional information:
Let us see some of the general characteristics of Neopentane.
- Neopentane is also known as 2,2-Dimethylpropane.
- It is similar to butane physically.
- It is highly flammable gas.
- Neopentane is low molecular weight alkane.
- It is a component of petroleum fuel mixture.
- It is less toxic.
So, the correct answer is “Option B”.
Note: The molecular weight that we have found is different from the molecular mass. The molecular weight is also known as the molar mass. The molecular weight is the weight or mass of one mole of the substance.
Complete Solution :
Vapour density of the hydrocarbon is given which is 36.
Molecular weight of the hydrocarbon \[ = 2 \times 36 = 72\]
Calculation of the molecular weight suggests that it is alkane.
The general formula of alkane is \[{C_n}{H_{2n + 2}}\].
Hydrocarbon A \[ = {C_n}{H_{2n + 2}} = 12n + 2n + 2 = 72\]
The hydrocarbon formed is \[{C_5}{H_{12}}\].
The hydrocarbon A on chlorination will give monochloro substituted product i.e. 1-Chloro-2,2-dimethylpropane.
The hydrocarbon A with the formula \[{C_5}{H_{12}}\] is Neopentane or 2,2-Dimethylpropane.

So, the correct answer is “Option B”. neo-pentane.
Additional information:
Let us see some of the general characteristics of Neopentane.
- Neopentane is also known as 2,2-Dimethylpropane.
- It is similar to butane physically.
- It is highly flammable gas.
- Neopentane is low molecular weight alkane.
- It is a component of petroleum fuel mixture.
- It is less toxic.
So, the correct answer is “Option B”.
Note: The molecular weight that we have found is different from the molecular mass. The molecular weight is also known as the molar mass. The molecular weight is the weight or mass of one mole of the substance.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




