
A group of students took an old shoe box and covered it with a black paper from all sides. They fixed a source of light (a torch) at one end of the box by making a hole in it and made another hole on the other side to view the light. They placed a milk sample contained in a beaker/tumbler in the box as shown in the Fig.2.4. They were amazed to see that milk taken in the tumbler was illuminated. They tried the same activity by taking a salt solution but found that light simply passed through it? Explain why the milk sample was illuminated. Name the phenomenon involved.
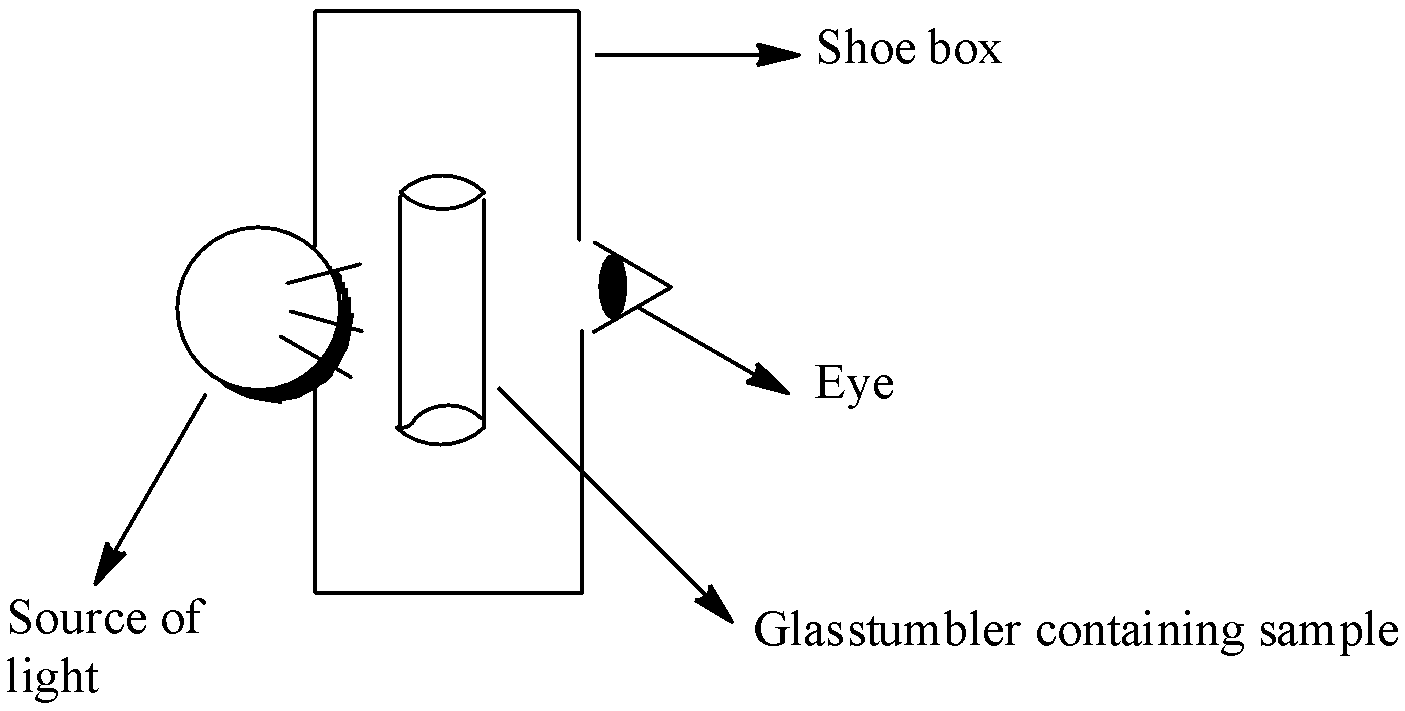
Fig. 2.4

Answer
531.6k+ views
Hint: If the size of the particles of the milk is in the range of the size between 1 nanometer to 1000 nanometer then these particles will cause the scattering of light which is a specific phenomenon caused by the particles only of this range.
Complete answer:
The students took two samples in this experiment that is milk and saltwater. We know that the salt solution is a homogenous solution that means the salt is completely soluble in water forming a true solution.
The size range of particles of the milk is in the range between the 1 nanometer to 1000 nanometer, which is the size range of colloidal particles. But the size range of the particles of the salt solution is in the range of less than 1 nanometer which is the size of true particles.
Hence the milk is a colloid and the salt solution is a true solution.
There is the illumination of light due to the Tyndall effect of the colloidal particles. The Tyndall effect is an optical effect, which may be defined by the scattering of the light by the particles. The true solutions do not give a Tyndall effect due to which there is no illumination of light.
Hence the phenomenon is due to the Tyndall effect of the colloidal particles.
Note:
There are other properties like the Brownian effect in which the colloidal particles move in the zig-zag pattern, coagulation in which the colloidal particles come together and form large particles, etc.
Complete answer:
The students took two samples in this experiment that is milk and saltwater. We know that the salt solution is a homogenous solution that means the salt is completely soluble in water forming a true solution.
The size range of particles of the milk is in the range between the 1 nanometer to 1000 nanometer, which is the size range of colloidal particles. But the size range of the particles of the salt solution is in the range of less than 1 nanometer which is the size of true particles.
Hence the milk is a colloid and the salt solution is a true solution.
There is the illumination of light due to the Tyndall effect of the colloidal particles. The Tyndall effect is an optical effect, which may be defined by the scattering of the light by the particles. The true solutions do not give a Tyndall effect due to which there is no illumination of light.
Hence the phenomenon is due to the Tyndall effect of the colloidal particles.
Note:
There are other properties like the Brownian effect in which the colloidal particles move in the zig-zag pattern, coagulation in which the colloidal particles come together and form large particles, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




