
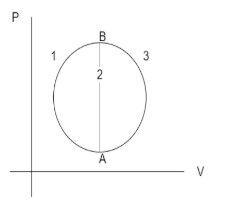
A gas undergoes A to B through three different processes $1,2,3$as shown in the figure. The heat supplied to the gas is ${Q_1},{Q_2},{Q_3}$ respectively, then
A) ${Q_1} = {Q_2} = {Q_3}$
B) ${Q_1} < {Q_2} < {Q_3}$
C) ${Q_1} > {Q_2} > {Q_3}$
D) ${Q_1} = {Q_3} > {Q_2}$

Answer
573.9k+ views
Hint:Recall that heat is the form of energy that is transferred between the objects when they are at different temperatures. Heat energy is also known as thermal energy. Heat energy is measured by using units in calories.
Step-By-Step Explanation:
Step I:
When the applied force and displacement are in the same direction, then work done is positive. The heat supplied in process $1$is positive. Therefore, the work done is also positive. The change of internal energy is given by the first law of thermodynamics.
Step II:
According to the first law of thermodynamics, change in the internal energy of a system is equal to the net transfer of heat in the system minus the net work done by the system. In equation form it can be written as
$\Delta U = Q - W$
Where W is the work done
Q is the heat energy transferred
$\Delta U$is the change in internal energy of the system
Step III:
Or For the first process,
${Q_1} = \Delta U + W$
Similarly, when the force and displacement are in a direction perpendicular to each other, then work done is said to be zero. In the process $2$, the work done is zero. The change in internal energy for this process can be written as
${Q_2} = \Delta U + zero$
Step IV:
The heat transfer in the process $3$is negative, because the force and displacement are in opposite directions to each other. So the change in internal energy of the system can be written as
${Q_3} = \Delta U - W$
Step V:
Therefore, the heat supplied will be in the order
${Q_1} > {Q_2} > {Q_3}$
$ \Rightarrow $Option C is the right answer.
Note:It is important to note that the heat transfer in a system depends on some factors. It depends on the mass of the substance and the temperature difference of the objects. The work done and the change in the internal energy of the system in thermodynamics depends on the initial and final state of the system.
Step-By-Step Explanation:
Step I:
When the applied force and displacement are in the same direction, then work done is positive. The heat supplied in process $1$is positive. Therefore, the work done is also positive. The change of internal energy is given by the first law of thermodynamics.
Step II:
According to the first law of thermodynamics, change in the internal energy of a system is equal to the net transfer of heat in the system minus the net work done by the system. In equation form it can be written as
$\Delta U = Q - W$
Where W is the work done
Q is the heat energy transferred
$\Delta U$is the change in internal energy of the system
Step III:
Or For the first process,
${Q_1} = \Delta U + W$
Similarly, when the force and displacement are in a direction perpendicular to each other, then work done is said to be zero. In the process $2$, the work done is zero. The change in internal energy for this process can be written as
${Q_2} = \Delta U + zero$
Step IV:
The heat transfer in the process $3$is negative, because the force and displacement are in opposite directions to each other. So the change in internal energy of the system can be written as
${Q_3} = \Delta U - W$
Step V:
Therefore, the heat supplied will be in the order
${Q_1} > {Q_2} > {Q_3}$
$ \Rightarrow $Option C is the right answer.
Note:It is important to note that the heat transfer in a system depends on some factors. It depends on the mass of the substance and the temperature difference of the objects. The work done and the change in the internal energy of the system in thermodynamics depends on the initial and final state of the system.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




