
A gas is taken through the cycle A B C A, as shown. What is the net work done by the gas?
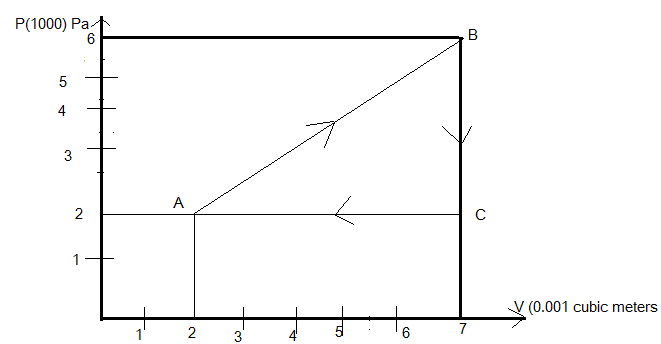
A. 3000J
B. 1000J
C. Zero
D. 2000J

Answer
589.8k+ views
Hint: The product of pressure and volume is represented by an area on the p-v diagram. The area under this curve tells us the work done during a thermodynamic process. In this process it can be found out by calculating the area of the curve this is a simple and quick method.
The curve showing the variation of volume of a substance along the x-axis and the variation of the pressure along the y-axis is the P-V diagram.
In this question first, find the total change in volume along the x-axis and change in pressure along the y-axis and then find the work done under the curve.
Complete step by step solution:
Given that the gas is going through the curve ABCA
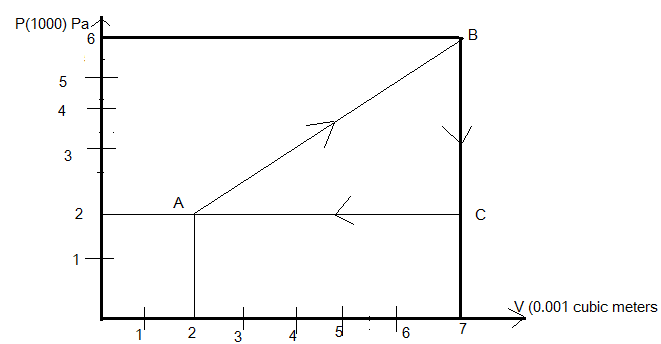
Where the change in volume takes place from \[2 \times {10^{ - 3}}{m^3}\]to \[7 \times {10^{ - 3}}{m^3}\]along positive x-axis and pressure changes from \[2 \times {10^5}Pa\] to \[6 \times {10^5}Pa\] along the y-direction.
Hence,
\[\vartriangle V = 5 \times {10^{ - 3}}{m^3}\]
\[\vartriangle P = 4 \times {10^5}Pa\]
So the area under the curve for the total work done will be equal to
Net work is done = area under the curve A B C
\[
W = \dfrac{1}{2} \times 5 \times {10^{ - 3}} \times 4 \times {10^5} \\
= \dfrac{1}{2} \times 5 \times 4 \times {10^2} \\
= 10 \times {10^2} \\
= 1000J \\
\]
Hence the total work is done by the gas under the curve =1000J
Option (B) is correct.
Note: Students should be careful in some cases where in some cases specific volume is plotted along the x-axis instead of volume; in such cases area under the curve represents work per unit mass of the working fluid given as J/Kg.
The curve showing the variation of volume of a substance along the x-axis and the variation of the pressure along the y-axis is the P-V diagram.
In this question first, find the total change in volume along the x-axis and change in pressure along the y-axis and then find the work done under the curve.
Complete step by step solution:
Given that the gas is going through the curve ABCA

Where the change in volume takes place from \[2 \times {10^{ - 3}}{m^3}\]to \[7 \times {10^{ - 3}}{m^3}\]along positive x-axis and pressure changes from \[2 \times {10^5}Pa\] to \[6 \times {10^5}Pa\] along the y-direction.
Hence,
\[\vartriangle V = 5 \times {10^{ - 3}}{m^3}\]
\[\vartriangle P = 4 \times {10^5}Pa\]
So the area under the curve for the total work done will be equal to
Net work is done = area under the curve A B C
\[
W = \dfrac{1}{2} \times 5 \times {10^{ - 3}} \times 4 \times {10^5} \\
= \dfrac{1}{2} \times 5 \times 4 \times {10^2} \\
= 10 \times {10^2} \\
= 1000J \\
\]
Hence the total work is done by the gas under the curve =1000J
Option (B) is correct.
Note: Students should be careful in some cases where in some cases specific volume is plotted along the x-axis instead of volume; in such cases area under the curve represents work per unit mass of the working fluid given as J/Kg.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




