
(a) Explain the process of nuclear fission and nuclear fusion by using the plot of binding energy per nucleon (BE/A) versus the mass number A.
(b) A radioactive isotope has a half-life year of 10 years. How long will it take for the activity to reduce to 3.125%?
Answer
587.7k+ views
Hint: Nuclear fission is a process in nuclear physics in which the nucleus of an atom splits into two or more smaller nuclei as fission products, and usually some by-product particles. Hence, fission is a form of elemental transmutation.
Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles (neutrons or protons). The difference in mass between the reactants and products is manifested as either the release or absorption of energy.
Complete step by step solution:
(a). Nuclear fission is a process in nuclear physics in which the nucleus of an atom splits into two or more smaller nuclei as fission products, and usually some by-product particles. Hence, fission is a form of elemental transmutation. When a heavy nucleus (A > 235 say) breaks into two lighter nuclei, the binding energy per nucleon increases i.e nucleons get more tightly bound. This implies that energy would be released in nuclear fission.
Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nucleus and subatomic particles (neutrons or protons). The difference in mass between the reactants and products is manifested as either the release or absorption of energy. When two very light nuclei join to form a heavy nucleus, the binding is energy per nucleon of fused heavier nucleus more than the binding energy per nucleon of lighter nuclei, so again energy would be released in nuclear fusion.
${}_1{H^2} + {}_1{H^2} \to {}_2H{e^4} + 26Mev$
The binding energy graph is as follows:
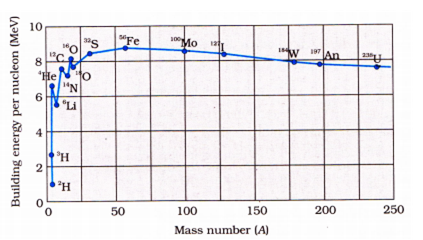
(b). Radioactive isotope, also called radioisotope, radionuclide, or radioactive nuclide, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess energy by spontaneously emitting radiation in the form of alpha, beta, and gamma rays.
Let the half life of the isotope be T years
And let the original amount of isotope be $N_0$
Therefore,
After decay, let the amount of the radioactive isotope be N
Then,
$\dfrac{N}{{N_0}} = 3.125\% = \dfrac{{3.125}}{{100}} = \dfrac{1}{{32}}$
But,
$\dfrac{N}{{N_0}} = {e^{ - \lambda t}}$ where $\lambda $ is the decay constant and t is the time
$
\therefore {e^{ - \lambda t}} = \dfrac{1}{{32}} \\
\Rightarrow - \lambda t = \ln 1 - \ln 32 \\
\Rightarrow - \lambda t = 0 - 3.4657 \\
\Rightarrow t = \dfrac{{3.4657}}{\lambda } \\
since\;\;\lambda = \dfrac{{0.693}}{t} \\
\therefore t = \dfrac{{3.466}}{{\dfrac{{0.693}}{T}}} \approx 5T\;\;years \\
$
Hence the isotope will take about 5T years to reduce to 3.125% of its original value.
Note: Radioactive decay follows first-order kinetics. Each radioactive nuclide has a characteristic, constant half-life (t1/2), the time required for half of the atoms in a sample to decay.
Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles (neutrons or protons). The difference in mass between the reactants and products is manifested as either the release or absorption of energy.
Complete step by step solution:
(a). Nuclear fission is a process in nuclear physics in which the nucleus of an atom splits into two or more smaller nuclei as fission products, and usually some by-product particles. Hence, fission is a form of elemental transmutation. When a heavy nucleus (A > 235 say) breaks into two lighter nuclei, the binding energy per nucleon increases i.e nucleons get more tightly bound. This implies that energy would be released in nuclear fission.
Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nucleus and subatomic particles (neutrons or protons). The difference in mass between the reactants and products is manifested as either the release or absorption of energy. When two very light nuclei join to form a heavy nucleus, the binding is energy per nucleon of fused heavier nucleus more than the binding energy per nucleon of lighter nuclei, so again energy would be released in nuclear fusion.
${}_1{H^2} + {}_1{H^2} \to {}_2H{e^4} + 26Mev$
The binding energy graph is as follows:

(b). Radioactive isotope, also called radioisotope, radionuclide, or radioactive nuclide, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess energy by spontaneously emitting radiation in the form of alpha, beta, and gamma rays.
Let the half life of the isotope be T years
And let the original amount of isotope be $N_0$
Therefore,
After decay, let the amount of the radioactive isotope be N
Then,
$\dfrac{N}{{N_0}} = 3.125\% = \dfrac{{3.125}}{{100}} = \dfrac{1}{{32}}$
But,
$\dfrac{N}{{N_0}} = {e^{ - \lambda t}}$ where $\lambda $ is the decay constant and t is the time
$
\therefore {e^{ - \lambda t}} = \dfrac{1}{{32}} \\
\Rightarrow - \lambda t = \ln 1 - \ln 32 \\
\Rightarrow - \lambda t = 0 - 3.4657 \\
\Rightarrow t = \dfrac{{3.4657}}{\lambda } \\
since\;\;\lambda = \dfrac{{0.693}}{t} \\
\therefore t = \dfrac{{3.466}}{{\dfrac{{0.693}}{T}}} \approx 5T\;\;years \\
$
Hence the isotope will take about 5T years to reduce to 3.125% of its original value.
Note: Radioactive decay follows first-order kinetics. Each radioactive nuclide has a characteristic, constant half-life (t1/2), the time required for half of the atoms in a sample to decay.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




