
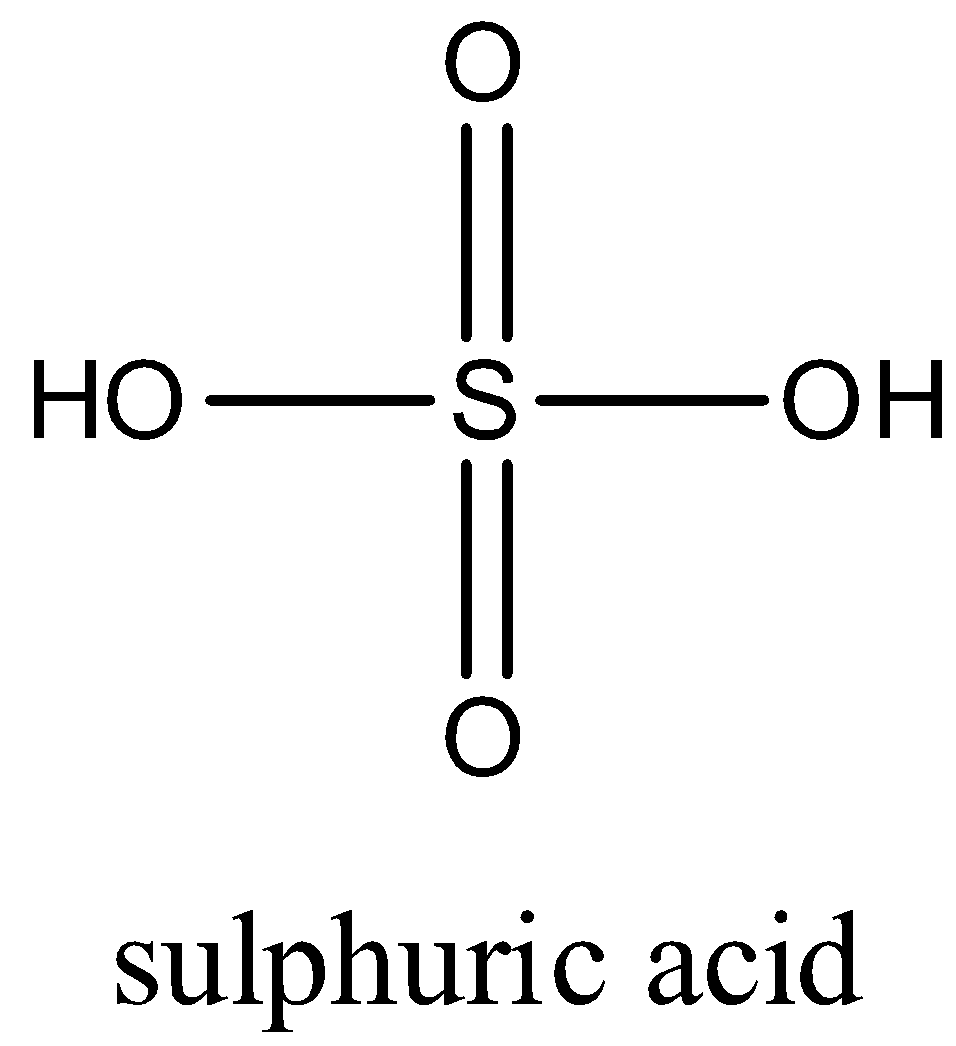
A. Draw the structure of sulphuric acid.
B. A sparkles current is passed through oxygen to prepare ozone. Why?
C. Bleaching action of Sulphur is a temporary action. Comment.
Answer
590.4k+ views
Hint: In sulphuric acid, Sulphur has two Sulphur oxygen double bonds and two Sulphur-OH bonds.
Pure and dry oxygen gas is passed. oxygen molecules dissociate and atomic oxygen is produced due to current. Under atmospheric oxygen, the colorless substance can gain its color back.
Complete step by step answer:
Sulphuric acid is an Oxoacid of Sulphur. In this, Sulphur is covalently bonded to two Oxo groups and two hydroxyl groups in a tetrahedral shape. Bond angle is 109.5.
The Electronic configuration of Sulphur is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{4}}$.
All four electrons of Sulphur are paired and Sulphur has no lone pairs.
-ozone is an allotropic form of oxygen.it is formed in presence of sunlight from atmospheric oxygen.
-preparation of ozone from oxygen is an endothermic process that is the reaction that absorbs heat.
-silent electrical discharge is used to prevent ozone’s decomposition.
-To prepare ozone in the laboratory, silent electric discharge is passed through dry oxygen, the electric current dissociates oxygen molecules to form oxygen atoms. Oxygen atoms combine with oxygen molecules to form ozone.
-Due to strong reducing properties of Sulphur dioxide, it is used in bleaching. Bleaching action of Sulphur is temporary because it involves reduction. Sulphur dioxide removes oxygen from colored substances and makes it colorless. Under the presence of atmospheric oxygen, material regains oxygen and its color.
Note: In bleaching action, Sulphur undergoes oxidation and colored substances undergoes reduction. Ozone acts as a powerful oxidizing agent because it can easily liberate nascent oxygen. Ozone is unstable than oxygen because its decomposition releases heat. The Valency of Sulphur is six.
Pure and dry oxygen gas is passed. oxygen molecules dissociate and atomic oxygen is produced due to current. Under atmospheric oxygen, the colorless substance can gain its color back.
Complete step by step answer:
Sulphuric acid is an Oxoacid of Sulphur. In this, Sulphur is covalently bonded to two Oxo groups and two hydroxyl groups in a tetrahedral shape. Bond angle is 109.5.
The Electronic configuration of Sulphur is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{4}}$.
All four electrons of Sulphur are paired and Sulphur has no lone pairs.

-ozone is an allotropic form of oxygen.it is formed in presence of sunlight from atmospheric oxygen.
-preparation of ozone from oxygen is an endothermic process that is the reaction that absorbs heat.
-silent electrical discharge is used to prevent ozone’s decomposition.
-To prepare ozone in the laboratory, silent electric discharge is passed through dry oxygen, the electric current dissociates oxygen molecules to form oxygen atoms. Oxygen atoms combine with oxygen molecules to form ozone.
-Due to strong reducing properties of Sulphur dioxide, it is used in bleaching. Bleaching action of Sulphur is temporary because it involves reduction. Sulphur dioxide removes oxygen from colored substances and makes it colorless. Under the presence of atmospheric oxygen, material regains oxygen and its color.
Note: In bleaching action, Sulphur undergoes oxidation and colored substances undergoes reduction. Ozone acts as a powerful oxidizing agent because it can easily liberate nascent oxygen. Ozone is unstable than oxygen because its decomposition releases heat. The Valency of Sulphur is six.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




