
a.) Draw cis and trans isomers of \[CHCl = CHCl\]. Also write their IUPAC names.
b.) Explain and give reasons which is more acidic: ethane or ethyne?
Answer
596.4k+ views
Hint: To answer this question, we first have to know that cis isomers are those which have same functional group on the same side while trans isomers have them on opposite side.
Coming to the second question, remember that acidity depends on the percentage s character.
Complete step by step solution:
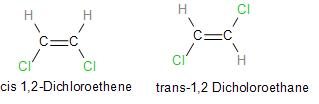
a.) We already know about stereoisomerism. Cis isomers are those in which two similar atoms lie on the same side of a double bond in the molecule and on the other hand, trans isomers have molecules with two similar atoms placed on opposite sides of a double bond.
Let us now look at the cis and trans isomer of dichloroethane:
b.) In ethyne, we see that the C atoms are sp hybridised which means 50% s character. As the s character increases the electronegativity increases hence ethyne attracts the electron pair of C-H bond towards itself and hydrogen ions are free. Thus ethyne is a strong acid as it can easily donate these hydrogen ions. In comparison to this, the carbon atom in ethane is \[s{p^3}\] hybridised which translates to 25% s character hence is less electronegative so it is difficult for it to donate hydrogen ions. Thus, ethyne is more acidic.
Note: We should note that Cis and trans isomers have the same molecular formula and molecular weight but differ in arrangement and due to the presence of strong attractive forces between atoms cis isomers have a high boiling point and melting point in comparison to trans isomers.
Coming to the second question, remember that acidity depends on the percentage s character.
Complete step by step solution:
a.) We already know about stereoisomerism. Cis isomers are those in which two similar atoms lie on the same side of a double bond in the molecule and on the other hand, trans isomers have molecules with two similar atoms placed on opposite sides of a double bond.
Let us now look at the cis and trans isomer of dichloroethane:

b.) In ethyne, we see that the C atoms are sp hybridised which means 50% s character. As the s character increases the electronegativity increases hence ethyne attracts the electron pair of C-H bond towards itself and hydrogen ions are free. Thus ethyne is a strong acid as it can easily donate these hydrogen ions. In comparison to this, the carbon atom in ethane is \[s{p^3}\] hybridised which translates to 25% s character hence is less electronegative so it is difficult for it to donate hydrogen ions. Thus, ethyne is more acidic.
Note: We should note that Cis and trans isomers have the same molecular formula and molecular weight but differ in arrangement and due to the presence of strong attractive forces between atoms cis isomers have a high boiling point and melting point in comparison to trans isomers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




