
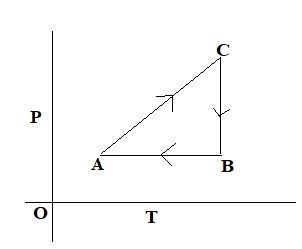
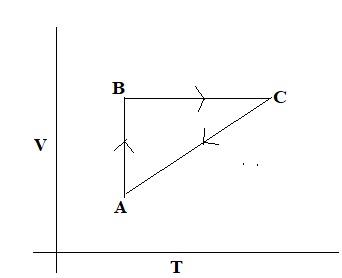
A cyclic process is shown in the P - T diagram: Which of the curves shows the same process on a V — T diagram?
A.
B.
C.
D.




Answer
558.6k+ views
Hint: Cyclic process is the process in which the initial and final state are the same i.e., the initial and final internal energies are equal when a system undergoes a cyclic process. The changes of different cures are compared to the question and the curve corresponding is found.
Complete step by step answer:
The above question can be understood from the gas laws, Boyle’s law and Charles's Law.
Boyle’s law states the relationship between the pressure and the volume of the gas at a constant temperature. It states that the volume of a gas is inversely proportional to the pressure of a gas at a constant temperature, expressed as
\[{{P}}\propto {\dfrac{1}{V}}\]
Charle’s law states that the volume of a gas is directly proportional to the temperature at constant pressure in a closed system, expressed as’
\[{{V }}\propto {{ T}}\]
The thermodynamic process in which temperature remains constant is called an isothermal process and process in which volume remains constant is called an isochoric process.
In the given question, the process from \[{{A}}\, \to \,{{B}}\], \[{{P}}\propto \,{{T}}\] and volume is constant (isochoric process). And from \[{{B}}\, \to \,{{C}}\] temperature is constant, and pressure decreases, from \[{{C}}\, \to \,{{A}}\], pressure is constant and temperature decreases. Comparing this with the options, in the option C, from \[{{A}}\, \to \,{{B}}\] volume is constant and temperature increases, and from \[{{B}}\, \to \,{{C}}\] temperature is constant, and volume increases, pressure decreases, \[{{C}}\, \to \,{{A}}\] temperature decreases.
So, the correct answer is Option C.
Additional information:
The internal energy change in any cyclic process is zero, the best example of a cyclic process is the Carnot engine.
Note: The relationship between temperature and pressure at constant volume is given by Gay-Lussac law. It states that at a constant volume, the pressure of the gas is directly proportional to the temperature for a given gas. The relationship between amount of gas and volume is given by Avogadro’s law. The law also states volume of gases is proportional to the number of the molecule in the ideal gas.
Complete step by step answer:
The above question can be understood from the gas laws, Boyle’s law and Charles's Law.
Boyle’s law states the relationship between the pressure and the volume of the gas at a constant temperature. It states that the volume of a gas is inversely proportional to the pressure of a gas at a constant temperature, expressed as
\[{{P}}\propto {\dfrac{1}{V}}\]
Charle’s law states that the volume of a gas is directly proportional to the temperature at constant pressure in a closed system, expressed as’
\[{{V }}\propto {{ T}}\]
The thermodynamic process in which temperature remains constant is called an isothermal process and process in which volume remains constant is called an isochoric process.
In the given question, the process from \[{{A}}\, \to \,{{B}}\], \[{{P}}\propto \,{{T}}\] and volume is constant (isochoric process). And from \[{{B}}\, \to \,{{C}}\] temperature is constant, and pressure decreases, from \[{{C}}\, \to \,{{A}}\], pressure is constant and temperature decreases. Comparing this with the options, in the option C, from \[{{A}}\, \to \,{{B}}\] volume is constant and temperature increases, and from \[{{B}}\, \to \,{{C}}\] temperature is constant, and volume increases, pressure decreases, \[{{C}}\, \to \,{{A}}\] temperature decreases.
So, the correct answer is Option C.
Additional information:
The internal energy change in any cyclic process is zero, the best example of a cyclic process is the Carnot engine.
Note: The relationship between temperature and pressure at constant volume is given by Gay-Lussac law. It states that at a constant volume, the pressure of the gas is directly proportional to the temperature for a given gas. The relationship between amount of gas and volume is given by Avogadro’s law. The law also states volume of gases is proportional to the number of the molecule in the ideal gas.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




