
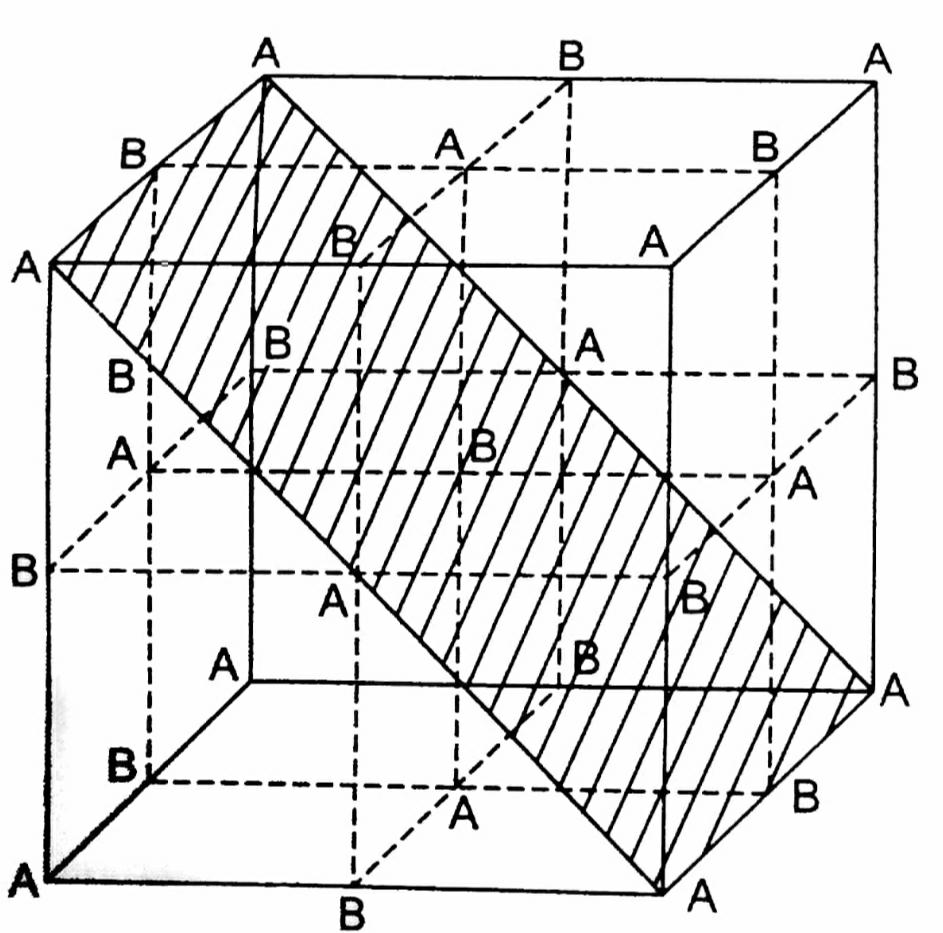
A crystal is made of particles A and B. A forms fcc packing and B occupies all the octahedral voids. If all the particles along the plane as shown in figure are removed, then the formula of the crystal will be.
\[\begin{array}{*{20}{l}}
{A) - {\text{ }}AB} \\
{B) - {\text{ }}{A_5}{B_7}} \\
{C) - {\text{ }}{A_7}{B_5}}
\end{array}\]
D)- None of these.

Answer
526.8k+ views
Hint: A crystal or crystalline solid is a solid material whose constituents are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. a piece of a homogeneous solid substance having a natural geometrically regular form with symmetrically arranged plane faces.
Complete step by step solution:
In FCC total 4 octahedral voids were present.
So, it contains 4 A and 4 B atoms but all the particles along the plane as shown in the figure given in the question are removed.
This plane contains 23 octahedral void atoms (1 at body center and 2 at edge center) and 23 lattice atoms (2 face centers and 4 corners).\[\begin{array}{*{20}{l}}
{A) - {\text{ }}AB} \\
{B) - {\text{ }}{A_5}{B_7}} \\
{C) - {\text{ }}{A_7}{B_5}}
\end{array}\]
So, the formula will be \[A3/2B3/2 = AB.\]
Hence, option A is the correct answer.
Note:
The void surrounded by six. sitting at the corners of a regular octahedron is called an octahedral void. The triangular voids in the second layer are just above the triangular voids in the first layer.A Lattice point is the position in the unit cell or in a crystal where the probability of finding an atom or an ion is the highest. In other words, the atoms or ions occupy the lattice points in a crystalline solid.
Complete step by step solution:
In FCC total 4 octahedral voids were present.
So, it contains 4 A and 4 B atoms but all the particles along the plane as shown in the figure given in the question are removed.
This plane contains 23 octahedral void atoms (1 at body center and 2 at edge center) and 23 lattice atoms (2 face centers and 4 corners).\[\begin{array}{*{20}{l}}
{A) - {\text{ }}AB} \\
{B) - {\text{ }}{A_5}{B_7}} \\
{C) - {\text{ }}{A_7}{B_5}}
\end{array}\]
So, the formula will be \[A3/2B3/2 = AB.\]
Hence, option A is the correct answer.
Note:
The void surrounded by six. sitting at the corners of a regular octahedron is called an octahedral void. The triangular voids in the second layer are just above the triangular voids in the first layer.A Lattice point is the position in the unit cell or in a crystal where the probability of finding an atom or an ion is the highest. In other words, the atoms or ions occupy the lattice points in a crystalline solid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




