
A compound (X) having molecular formula ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}$is hydrolysed by water in presence of an acid to give a carboxylic acid(Y) and an alcohol (Z). (Z) On oxidation with chromic acid gives (Y). (X), (Y) and (Z) are:
(A)$\,\text{X=}\,\text{C}{{\text{H}}_{\text{3}}}\text{COOC}{{\text{H}}_{\text{3}}}\,\,\text{Y=}\,\text{C}{{\text{H}}_{\text{3}}}\text{COOH}\,\,\,\text{Z=}\,\text{C}{{\text{H}}_{\text{3}}}\text{OH}$
(B) $\,\text{X=}\,\text{C}{{\text{H}}_{\text{3}}}\text{COO}{{\text{C}}_{2}}{{\text{H}}_{5}}\,\,\text{Y=}\,\text{C}{{\text{H}}_{\text{3}}}\text{COOH}\,\,\,\text{Z=}\,{{\text{C}}_{2}}{{\text{H}}_{5}}\text{OH}$
(C) $\,\text{X=}\,{{\text{C}}_{2}}{{\text{H}}_{5}}\text{COOC}{{\text{H}}_{\text{3}}}\,\,\text{Y=}\,{{\text{C}}_{2}}{{\text{H}}_{5}}\text{COOH}\,\,\,\text{Z=}\,{{\text{C}}_{2}}{{\text{H}}_{5}}\text{OH}$
(D) $\,\text{X=}\,\text{C}{{\text{H}}_{\text{3}}}\text{COO}{{\text{C}}_{2}}{{\text{H}}_{5}}\,\,\text{Y=}\,{{\text{C}}_{2}}{{\text{H}}_{5}}\text{COOH}\,\,\,\text{Z=}\,\text{C}{{\text{H}}_{\text{3}}}\text{OH}$
Answer
568.8k+ views
Hint:The unsaturation factor of this given formula is one so this compound must have one double bond. This compound has two oxygen atoms so it should be either acid or its functional group isomer ester.
Ester on hydrolysis gives acid and alcohol.
Chromic acid is a strong oxidising agent; only 1- alkanol gives acid on oxidation.
Complete answer:
Ethyl ethanoate is the ester which gives acetic acid and ethanol on hydrolysis in the presence of a strong acid. The hydrolysis of ester is mainly nucleophilic addition and elimination type of reaction. This reaction occurs in following manner-
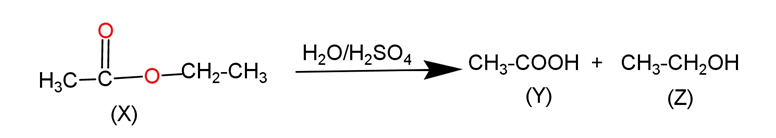
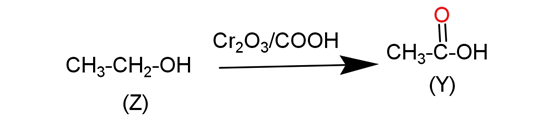
Ethyl alcohol is an ${{1}^{\circ }}$ alcohol which on oxidation in the presence of chromic acid gives acid with the same number of carbon in the parent chain. This reaction is occurs in following manner-
Since, in this given question compound$\,\text{X=}\,\text{C}{{\text{H}}_{\text{3}}}\text{COO}{{\text{C}}_{2}}{{\text{H}}_{5}}$, compound $\text{Y=}\,\text{C}{{\text{H}}_{\text{3}}}\text{COOH}$and compound$\,\text{Z=}\,{{\text{C}}_{2}}{{\text{H}}_{5}}\text{OH}$.
So, option (B) will be the correct option.
Note:
During the preparation of esters a carboxylic acid and an alcohol in the presence of an acid catalyst, the water or the ester should be removed as soon formed because if either water or ester is not removed as soon as it is formed, then it reacts to give back the reactants as the reaction is reversible.
Ester on hydrolysis gives acid and alcohol.
Chromic acid is a strong oxidising agent; only 1- alkanol gives acid on oxidation.
Complete answer:
Ethyl ethanoate is the ester which gives acetic acid and ethanol on hydrolysis in the presence of a strong acid. The hydrolysis of ester is mainly nucleophilic addition and elimination type of reaction. This reaction occurs in following manner-

Ethyl alcohol is an ${{1}^{\circ }}$ alcohol which on oxidation in the presence of chromic acid gives acid with the same number of carbon in the parent chain. This reaction is occurs in following manner-

Since, in this given question compound$\,\text{X=}\,\text{C}{{\text{H}}_{\text{3}}}\text{COO}{{\text{C}}_{2}}{{\text{H}}_{5}}$, compound $\text{Y=}\,\text{C}{{\text{H}}_{\text{3}}}\text{COOH}$and compound$\,\text{Z=}\,{{\text{C}}_{2}}{{\text{H}}_{5}}\text{OH}$.
So, option (B) will be the correct option.
Note:
During the preparation of esters a carboxylic acid and an alcohol in the presence of an acid catalyst, the water or the ester should be removed as soon formed because if either water or ester is not removed as soon as it is formed, then it reacts to give back the reactants as the reaction is reversible.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




