
A compound formed by elements A and B crystallizes in the cubic structure where A atoms are at the corners of the cube B atoms are at the face centres. The formula of the compound is:
(A) $A{B_3}$
(B) $AB$
(C) ${A_3}B$
(D) ${A_2}{B_2}$
Answer
582.3k+ views
Hint:Solid compounds are mostly made up of crystal. Simplest crystals are called simple cubic structures. The simple cubic structure can either be body-centered cubic structure or face-centered cubic structure which have an extra atom at center of each cube or each face of the cube respectively.
Complete step by step answer: For determining the formula of the given compound, we use some information given below:
As, A are the atoms that are present at the corners of the cube and B are the atoms present at the face centers of the cubes.
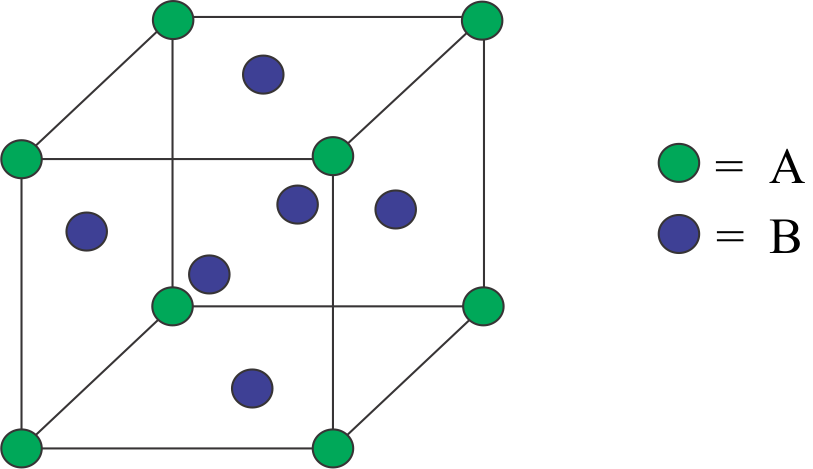
We must know that all atoms that are present in the corner of the crystal lattice have their $\dfrac{1}{8}$ portion in the lattice.
Similarly all the atoms that are present in the ace center of the crystal lattice have their $\dfrac{1}{2}$ portion in the lattice.
We can collate the formulas:
Atom A at the corner will contribute to unit cell =$\dfrac{1}{8}$ portion.
Atoms present at corners of lattice =8
Therefore, number of atoms of A in the unit cell =$8 \times \dfrac{1}{8} = 1$
Similarly, atoms present at the face center will contribute to the unit cell =$\dfrac{1}{2}$ portion.
And atoms present at face center=6
Therefore, number of atoms of b in the unit cell =$6 \times \dfrac{1}{2}$
Hence by the above information we can clued that the formula of the compound would be ${A_1}{B_3}$ OR $A{B_{3.}}$
Hence the correct option is A.
Additional information:-
Crystal lattice of a compound can be defined as the orderly arrangement of atoms and ions in an ordered manner in space which extends in all directions. It is useful in determining the various properties of the respective compounds like ductility, deformability, brittleness, creep and behaviour of compounds under various conditions.
Note:For the calculation of the formula of the compound, we need to know two values, one is the number of atoms present at the lattice corner of the face center.
Another value is the contribution of a particular atom is the unit cell. Only then the number of atoms of that element and formula of compound can be calculated.
Complete step by step answer: For determining the formula of the given compound, we use some information given below:
As, A are the atoms that are present at the corners of the cube and B are the atoms present at the face centers of the cubes.

We must know that all atoms that are present in the corner of the crystal lattice have their $\dfrac{1}{8}$ portion in the lattice.
Similarly all the atoms that are present in the ace center of the crystal lattice have their $\dfrac{1}{2}$ portion in the lattice.
We can collate the formulas:
Atom A at the corner will contribute to unit cell =$\dfrac{1}{8}$ portion.
Atoms present at corners of lattice =8
Therefore, number of atoms of A in the unit cell =$8 \times \dfrac{1}{8} = 1$
Similarly, atoms present at the face center will contribute to the unit cell =$\dfrac{1}{2}$ portion.
And atoms present at face center=6
Therefore, number of atoms of b in the unit cell =$6 \times \dfrac{1}{2}$
Hence by the above information we can clued that the formula of the compound would be ${A_1}{B_3}$ OR $A{B_{3.}}$
Hence the correct option is A.
Additional information:-
Crystal lattice of a compound can be defined as the orderly arrangement of atoms and ions in an ordered manner in space which extends in all directions. It is useful in determining the various properties of the respective compounds like ductility, deformability, brittleness, creep and behaviour of compounds under various conditions.
Note:For the calculation of the formula of the compound, we need to know two values, one is the number of atoms present at the lattice corner of the face center.
Another value is the contribution of a particular atom is the unit cell. Only then the number of atoms of that element and formula of compound can be calculated.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




