
A compound contains $28$ percent of nitrogen and $72$ percent of a metal by weight. Three atoms of the metal combine with two atoms of $N$ . Find the atomic weight of the metal :
$24g$
$12g$
$72g$
$42g$
Answer
559.5k+ views
Hint: Given that $28%$ of $N$ and $72%$ of Metal in total its $3$ atoms where Nitrogen are $2$ atoms from $3$. Finding the Equivalent weight of compound and then applying with given $%$ of both metal and then Equivalent weight with Valency will give the atomic weight of Metal.
Complete step by step answer:
According to problem, three atoms of $M$ combine with $2$ atoms of $N$
Thus, the formula of compound $={{M}_{3}}{{N}_{2}}$
Equivalent Weight of $N=\dfrac{14}{3}$ ( Valency of $N$ in compound is $3$)
$\therefore 28g$ $N$ combines with $=72g$ metal
$\therefore \dfrac{14}{3}$ $N$ combines with $=\dfrac{72}{28}\times \dfrac{14}{3} = 12g$ metal
$\therefore $ Equation weight of metal $=12g$
Atomic weight of metal $=$ Equivalent weight $\times $ Valency
$=12\times 2$
$=24$[Valency]
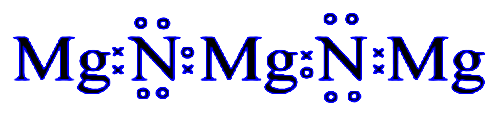
Additional Information: The metal is magnesium as its getting confirmed by its property and the compound in question is magnesium nitride ${{M}_{3}}{{N}_{2}}$
Note: Remember we have to keep this rule before solving, $Mg$ has $2$ valence electrons and loses these $2$ valence electrons to form $M{{g}^{2+}}$ion. Similarly, $N$ has $5$ valence electrons. It gains $3$ valence electrons to form ${{N}^{3-}}$ ion. Alternatively to balance charges, $3M{{g}^{2+}}$ ion combines with two ${{N}^{3-}}$ ions to form ${{M}_{3}}{{N}_{2}}$.
Complete step by step answer:
According to problem, three atoms of $M$ combine with $2$ atoms of $N$
Thus, the formula of compound $={{M}_{3}}{{N}_{2}}$
Equivalent Weight of $N=\dfrac{14}{3}$ ( Valency of $N$ in compound is $3$)
$\therefore 28g$ $N$ combines with $=72g$ metal
$\therefore \dfrac{14}{3}$ $N$ combines with $=\dfrac{72}{28}\times \dfrac{14}{3} = 12g$ metal
$\therefore $ Equation weight of metal $=12g$
Atomic weight of metal $=$ Equivalent weight $\times $ Valency
$=12\times 2$
$=24$[Valency]
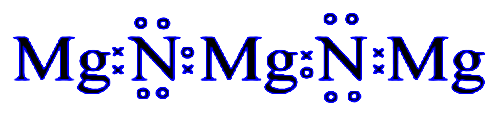
Additional Information: The metal is magnesium as its getting confirmed by its property and the compound in question is magnesium nitride ${{M}_{3}}{{N}_{2}}$
Note: Remember we have to keep this rule before solving, $Mg$ has $2$ valence electrons and loses these $2$ valence electrons to form $M{{g}^{2+}}$ion. Similarly, $N$ has $5$ valence electrons. It gains $3$ valence electrons to form ${{N}^{3-}}$ ion. Alternatively to balance charges, $3M{{g}^{2+}}$ ion combines with two ${{N}^{3-}}$ ions to form ${{M}_{3}}{{N}_{2}}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




