
A complex involving ${{d}^{2}}s{{p}^{3}}$ hybridisation is :
(A) a square planar geometry
(B) a tetrahedral geometry
(C) an octahedral geometry
(D) trigonal planar geometry
Answer
584.4k+ views
Hint: Hybridization is calculated in accordance with the Valence bond theory. Take an example of a metal complex whose hybridization is ${{d}^{2}}s{{p}^{3}}$. Now draw the expanded structure of the metal complex to identify its geometry and thus answer the question.
Complete step by step solution:
According to valence bond theory, electrons in a molecule occupy atomic orbitals and not molecular orbitals. The atomic orbitals overlap on the bond formation and the strength of the bond depends on the extent of overlap.
Postulates of Valence Bond Theory:
-Covalent bonds are formed when two valence orbitals belonging to two different atoms overlap onto each other. Due to overlapping the electron density in that region increases, thereby increasing the stability of the molecule thus formed.
-The presence of many unpaired electrons in the valence shell of an atom enables the atoms to form multiple bonds with each other. However, the paired electrons present in the valent shell do not take part in the formation of chemical bonds.
-Covalent bonds are directional and parallel to the region corresponding to atomic orbitals that are going to overlap.
-Sigma bonds and pi bonds differ in the pattern that the atomic orbitals overlap in, i.e. sigma bonds undergo head-on overlap however pi bonds undergo sideways overlapping.
We will now take an example of a complex that exhibits the hybridization, ${{d}^{2}}s{{p}^{3}}$.
Example: ${{\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$
The structure of the above compound is:
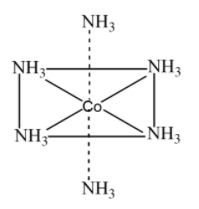
The above complex has 6 ligands attached to it in a 3d space. The geometry of this complex is thus octahedral. We can now generalize that a compound with hybridization ${{d}^{2}}s{{p}^{3}}$ will have octahedral geometry.
Therefore, the correct answer is option (C).
Note: Valence bond theory was successful in determining the hybridization of atoms in a molecule. However, the theory had some limitations, like:
-Unable to explain the tetravalency of carbon
-No insight or information on the energies of electrons
-Incorrect assumption that electrons are localized in specific areas only
-No distinction between weak and strong ligands ( hybridization of complex compounds)
Complete step by step solution:
According to valence bond theory, electrons in a molecule occupy atomic orbitals and not molecular orbitals. The atomic orbitals overlap on the bond formation and the strength of the bond depends on the extent of overlap.
Postulates of Valence Bond Theory:
-Covalent bonds are formed when two valence orbitals belonging to two different atoms overlap onto each other. Due to overlapping the electron density in that region increases, thereby increasing the stability of the molecule thus formed.
-The presence of many unpaired electrons in the valence shell of an atom enables the atoms to form multiple bonds with each other. However, the paired electrons present in the valent shell do not take part in the formation of chemical bonds.
-Covalent bonds are directional and parallel to the region corresponding to atomic orbitals that are going to overlap.
-Sigma bonds and pi bonds differ in the pattern that the atomic orbitals overlap in, i.e. sigma bonds undergo head-on overlap however pi bonds undergo sideways overlapping.
We will now take an example of a complex that exhibits the hybridization, ${{d}^{2}}s{{p}^{3}}$.
Example: ${{\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$
The structure of the above compound is:

The above complex has 6 ligands attached to it in a 3d space. The geometry of this complex is thus octahedral. We can now generalize that a compound with hybridization ${{d}^{2}}s{{p}^{3}}$ will have octahedral geometry.
Therefore, the correct answer is option (C).
Note: Valence bond theory was successful in determining the hybridization of atoms in a molecule. However, the theory had some limitations, like:
-Unable to explain the tetravalency of carbon
-No insight or information on the energies of electrons
-Incorrect assumption that electrons are localized in specific areas only
-No distinction between weak and strong ligands ( hybridization of complex compounds)
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




