
(a) Complete the following chemical equations:
(i) ${\text{NaOH (hot and conc}}{\text{.)}} + {\text{C}}{{\text{l}}_2} \to $
(ii) ${\text{Xe}}{{\text{F}}_{\text{4}}} + {{\text{O}}_2}{{\text{F}}_2} \to $
(b) Draw the structures of the following molecules:
(i) ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$
(ii) ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$
(iii) ${\text{XeO}}{{\text{F}}_{\text{4}}}$
Answer
578.4k+ views
Hint: Draw the structures of the molecules by counting the valence electrons for the molecule. The valence electrons are donated by dots around the atoms and are called electron dot structures.
Complete step by step answer:
(a)(i) Complete the chemical equation ${\text{NaOH (hot and conc}}{\text{.)}} + {\text{C}}{{\text{l}}_2} \to $:
Hot and concentrated sodium hydroxide reacts with chlorine to form sodium chloride, sodium chlorate and water.
The chemical formula for sodium chloride is ${\text{NaCl}}$ and the chemical formula for sodium chlorate is ${\text{NaCl}}{{\text{O}}_3}$.
Thus, the chemical equation is as follows:
${\text{NaOH (hot and conc}}{\text{.)}} + {\text{C}}{{\text{l}}_2} \to {\text{NaCl}} + {\text{NaCl}}{{\text{O}}_3} + {{\text{H}}_2}{\text{O}}$
The balanced chemical equation is as follows:
${\text{6NaOH (hot and conc}}{\text{.)}} + 3{\text{C}}{{\text{l}}_2} \to 5{\text{NaCl}} + {\text{NaCl}}{{\text{O}}_3} + 3{{\text{H}}_2}{\text{O}}$
(ii) Complete the chemical equation ${\text{Xe}}{{\text{F}}_{\text{4}}} + {{\text{O}}_2}{{\text{F}}_2} \to $:
Xenon tetrafluoride reacts with fluorine dioxide to form xenon hexafluoride and oxygen gas.
The chemical formula for xenon hexafluoride is ${\text{Xe}}{{\text{F}}_6}$.
Thus, the chemical equation is as follows:
${\text{Xe}}{{\text{F}}_{\text{4}}} + {{\text{O}}_2}{{\text{F}}_2} \to {\text{Xe}}{{\text{F}}_{\text{6}}} + {{\text{O}}_2}$
(b)(ii) Draw the structures of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$:
Calculate the valence electrons of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ molecule as follows:
The valence electrons of hydrogen are one, phosphorous are five and oxygen are six. Thus,
Valence electrons of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$
$ = \left( {3 \times {\text{Valence electrons of H}}} \right) + \left( {1 \times {\text{Valence electrons of P}}} \right) + \left( {2 \times {\text{Valence electrons of O}}} \right)$
$ = \left( {3 \times 1} \right) + \left( {1 \times 5} \right) + \left( {2 \times 6} \right)$
= 3 + 5 + 12
Valence electrons of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ $ = {\text{20}}$
Draw the Lewis structure of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ as follows:
The structure of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ is,
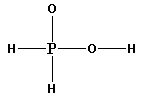
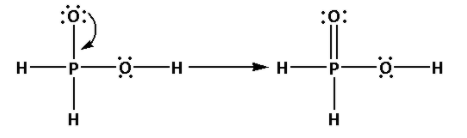
As five bonds are formed, ten electrons are involved in bonding. Thus, the remaining electrons are,
Remaining electrons = 20 - 10 = 10
Place the remaining 10 electrons around the oxygen atoms such that all the oxygen atoms complete their octets.
Thus, the structure of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ is as follows:
(ii) Draw the structures of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$:
Calculate the valence electrons of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ molecule as follows:
The valence electrons of hydrogen are one, sulphur are six and oxygen are six. Thus,
Valence electrons of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$
$ = \left( {2 \times {\text{Valence electrons of H}}} \right) + \left( {2 \times {\text{Valence electrons of S}}} \right) + \left( {7 \times {\text{Valence electrons of O}}} \right)$
$ = \left( {2 \times 1} \right) + \left( {2 \times 6} \right) + \left( {7 \times 6} \right)$
= 2 + 12 + 42
Valence electrons of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ $ = {\text{56}}$
Draw the Lewis structure of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ as follows:
The structure of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ is,
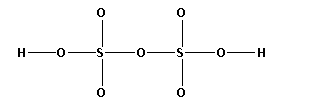
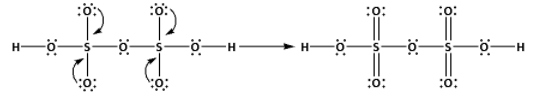
As ten bonds are formed, twenty electrons are involved in bonding. Thus, the remaining electrons are,
Remaining electrons = 56 - 20 = 36
Place the remaining 36 electrons around the oxygen atoms such that all the oxygen atoms complete their octets.
Thus, the structure of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ is as follows:
(iii) Draw the structures of ${\text{XeO}}{{\text{F}}_{\text{4}}}$:
Calculate the valence electrons of ${\text{XeO}}{{\text{F}}_{\text{4}}}$ molecule as follows:
The valence electrons of xenon are eight, oxygen are six and fluorine are seven. Thus,
Valence electrons of ${\text{XeO}}{{\text{F}}_{\text{4}}}$
$ = \left( {1 \times {\text{Valence electrons of Xe}}} \right) + \left( {1 \times {\text{Valence electrons of O}}} \right) + \left( {4 \times {\text{Valence electrons of F}}} \right)$
$ = \left( {1 \times 8} \right) + \left( {1 \times 6} \right) + \left( {4 \times 7} \right)$
= 8 + 6 + 28
Valence electrons of ${\text{XeO}}{{\text{F}}_{\text{4}}}$ $ = {\text{42}}$
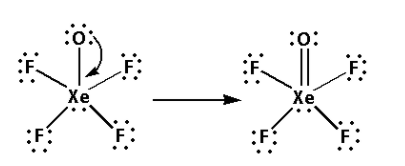
Draw the Lewis structure of ${\text{XeO}}{{\text{F}}_{\text{4}}}$ as follows:
The structure of ${\text{XeO}}{{\text{F}}_{\text{4}}}$ is,
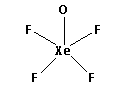
As five bonds are formed, ten electrons are involved in bonding. Thus, the remaining electrons are,
Remaining electrons = 42 - 10 = 32
Place the remaining 32 electrons around the oxygen atoms such that all the oxygen atoms complete their octets.
Thus, the structure of ${\text{XeO}}{{\text{F}}_{\text{4}}}$ is as follows:
Note: Steps to determine the structure of a molecule are as follows:
- Determine the number of total valence electrons. (The number of valence electrons of an atom is equal to the number of the group in which the atom lies.)
- Determine the number of electrons involved in bonding.
- Determine the number of remaining electrons.
- Draw the Lewis structure.
Complete step by step answer:
(a)(i) Complete the chemical equation ${\text{NaOH (hot and conc}}{\text{.)}} + {\text{C}}{{\text{l}}_2} \to $:
Hot and concentrated sodium hydroxide reacts with chlorine to form sodium chloride, sodium chlorate and water.
The chemical formula for sodium chloride is ${\text{NaCl}}$ and the chemical formula for sodium chlorate is ${\text{NaCl}}{{\text{O}}_3}$.
Thus, the chemical equation is as follows:
${\text{NaOH (hot and conc}}{\text{.)}} + {\text{C}}{{\text{l}}_2} \to {\text{NaCl}} + {\text{NaCl}}{{\text{O}}_3} + {{\text{H}}_2}{\text{O}}$
The balanced chemical equation is as follows:
${\text{6NaOH (hot and conc}}{\text{.)}} + 3{\text{C}}{{\text{l}}_2} \to 5{\text{NaCl}} + {\text{NaCl}}{{\text{O}}_3} + 3{{\text{H}}_2}{\text{O}}$
(ii) Complete the chemical equation ${\text{Xe}}{{\text{F}}_{\text{4}}} + {{\text{O}}_2}{{\text{F}}_2} \to $:
Xenon tetrafluoride reacts with fluorine dioxide to form xenon hexafluoride and oxygen gas.
The chemical formula for xenon hexafluoride is ${\text{Xe}}{{\text{F}}_6}$.
Thus, the chemical equation is as follows:
${\text{Xe}}{{\text{F}}_{\text{4}}} + {{\text{O}}_2}{{\text{F}}_2} \to {\text{Xe}}{{\text{F}}_{\text{6}}} + {{\text{O}}_2}$
(b)(ii) Draw the structures of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$:
Calculate the valence electrons of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ molecule as follows:
The valence electrons of hydrogen are one, phosphorous are five and oxygen are six. Thus,
Valence electrons of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$
$ = \left( {3 \times {\text{Valence electrons of H}}} \right) + \left( {1 \times {\text{Valence electrons of P}}} \right) + \left( {2 \times {\text{Valence electrons of O}}} \right)$
$ = \left( {3 \times 1} \right) + \left( {1 \times 5} \right) + \left( {2 \times 6} \right)$
= 3 + 5 + 12
Valence electrons of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ $ = {\text{20}}$
Draw the Lewis structure of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ as follows:
The structure of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ is,

As five bonds are formed, ten electrons are involved in bonding. Thus, the remaining electrons are,
Remaining electrons = 20 - 10 = 10
Place the remaining 10 electrons around the oxygen atoms such that all the oxygen atoms complete their octets.
Thus, the structure of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ is as follows:

(ii) Draw the structures of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$:
Calculate the valence electrons of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ molecule as follows:
The valence electrons of hydrogen are one, sulphur are six and oxygen are six. Thus,
Valence electrons of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$
$ = \left( {2 \times {\text{Valence electrons of H}}} \right) + \left( {2 \times {\text{Valence electrons of S}}} \right) + \left( {7 \times {\text{Valence electrons of O}}} \right)$
$ = \left( {2 \times 1} \right) + \left( {2 \times 6} \right) + \left( {7 \times 6} \right)$
= 2 + 12 + 42
Valence electrons of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ $ = {\text{56}}$
Draw the Lewis structure of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ as follows:
The structure of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ is,

As ten bonds are formed, twenty electrons are involved in bonding. Thus, the remaining electrons are,
Remaining electrons = 56 - 20 = 36
Place the remaining 36 electrons around the oxygen atoms such that all the oxygen atoms complete their octets.
Thus, the structure of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}$ is as follows:

(iii) Draw the structures of ${\text{XeO}}{{\text{F}}_{\text{4}}}$:
Calculate the valence electrons of ${\text{XeO}}{{\text{F}}_{\text{4}}}$ molecule as follows:
The valence electrons of xenon are eight, oxygen are six and fluorine are seven. Thus,
Valence electrons of ${\text{XeO}}{{\text{F}}_{\text{4}}}$
$ = \left( {1 \times {\text{Valence electrons of Xe}}} \right) + \left( {1 \times {\text{Valence electrons of O}}} \right) + \left( {4 \times {\text{Valence electrons of F}}} \right)$
$ = \left( {1 \times 8} \right) + \left( {1 \times 6} \right) + \left( {4 \times 7} \right)$
= 8 + 6 + 28
Valence electrons of ${\text{XeO}}{{\text{F}}_{\text{4}}}$ $ = {\text{42}}$
Draw the Lewis structure of ${\text{XeO}}{{\text{F}}_{\text{4}}}$ as follows:
The structure of ${\text{XeO}}{{\text{F}}_{\text{4}}}$ is,

As five bonds are formed, ten electrons are involved in bonding. Thus, the remaining electrons are,
Remaining electrons = 42 - 10 = 32
Place the remaining 32 electrons around the oxygen atoms such that all the oxygen atoms complete their octets.
Thus, the structure of ${\text{XeO}}{{\text{F}}_{\text{4}}}$ is as follows:

Note: Steps to determine the structure of a molecule are as follows:
- Determine the number of total valence electrons. (The number of valence electrons of an atom is equal to the number of the group in which the atom lies.)
- Determine the number of electrons involved in bonding.
- Determine the number of remaining electrons.
- Draw the Lewis structure.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




