
A certain alkene produces the product shown, when subjected to ozonolysis followed by oxidative work-up. What is the structure of alkene?
$ Alkene\xrightarrow[{{H_2}{O_2}}]{{{O_3}}}HOOCC{H_2}C{H_2}COOH + C{H_3}COC{H_3} + HCOOH $
(A)
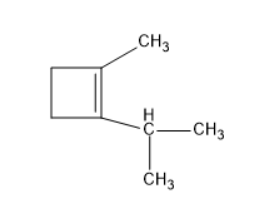
(B) $ {\left( {C{H_3}} \right)_2}C = CHC{H_2}C{H_2}CH = C{H_2} $
(C) $ C{H_3}CH = CHC{H_2}C{H_2}CH = CHC{H_2} $
(D) $ C{H_3}C{H_2}CH = C = CHC{H_2}CH = C{H_2} $

Answer
494.4k+ views
Hint: Alkenes consist of double bonded carbon and carbon atoms. When alkenes are subjected to ozonolysis carbonyl compounds will be formed at the double bond positions. Further oxidative work up converts aldehydes into carboxylic compounds, whereas ketones will remain like that.
Complete answer:
Alkenes are unsaturated hydrocarbons consisting of carbon and hydrogen atoms. There will be one or more double bonded carbon atoms in alkenes. When alkenes are subjected to ozonolysis the double bonds will convert into carbonyl compounds which are aldehydes and ketones.
At the position of double bond, the carbonyl compounds will be formed. Aldehydes will convert into carboxylic acid when ozonolysis is followed by oxidation.
In the given option acetone is formed. Thus, the alkenes should consist of an isopropyl group. In the given options, option B has the isopropyl group.
At the position of double bond, the carbonyl compounds will be formed upon ozonolysis.
$ {\left( {C{H_3}} \right)_2}C = CHC{H_2}C{H_2}CH = C{H_2}\xrightarrow{{{O_3}}}C{H_3}COC{H_3} + OHCC{H_2}C{H_2}CHO + HCHO $
The above products were formed upon ozonolysis and oxidative work up of the above products the aldehydes convert into carboxylic acids.
The oxidative work up the reagent is hydrogen peroxide.
$ C{H_3}COC{H_3} + OHCC{H_2}C{H_2}CHO + HCHO\xrightarrow{{{H_2}{O_2}}}C{H_3}COC{H_3} + HOOCC{H_2}C{H_2}COOH + HCOOH $
Thus, the alkene is $ {\left( {C{H_3}} \right)_2}C = CHC{H_2}C{H_2}CH = C{H_2} $
Option B is the correct one.
Note:
Ozonolysis means the addition of ozone molecules to alkenes. Ozonide will be formed in the reaction. Later the oxidative work up means the oxidation takes place. But only aldehydes convert into carboxylic acids. The ketones will not convert into carboxylic acids is an important point.
Complete answer:
Alkenes are unsaturated hydrocarbons consisting of carbon and hydrogen atoms. There will be one or more double bonded carbon atoms in alkenes. When alkenes are subjected to ozonolysis the double bonds will convert into carbonyl compounds which are aldehydes and ketones.
At the position of double bond, the carbonyl compounds will be formed. Aldehydes will convert into carboxylic acid when ozonolysis is followed by oxidation.
In the given option acetone is formed. Thus, the alkenes should consist of an isopropyl group. In the given options, option B has the isopropyl group.
At the position of double bond, the carbonyl compounds will be formed upon ozonolysis.
$ {\left( {C{H_3}} \right)_2}C = CHC{H_2}C{H_2}CH = C{H_2}\xrightarrow{{{O_3}}}C{H_3}COC{H_3} + OHCC{H_2}C{H_2}CHO + HCHO $
The above products were formed upon ozonolysis and oxidative work up of the above products the aldehydes convert into carboxylic acids.
The oxidative work up the reagent is hydrogen peroxide.
$ C{H_3}COC{H_3} + OHCC{H_2}C{H_2}CHO + HCHO\xrightarrow{{{H_2}{O_2}}}C{H_3}COC{H_3} + HOOCC{H_2}C{H_2}COOH + HCOOH $
Thus, the alkene is $ {\left( {C{H_3}} \right)_2}C = CHC{H_2}C{H_2}CH = C{H_2} $
Option B is the correct one.
Note:
Ozonolysis means the addition of ozone molecules to alkenes. Ozonide will be formed in the reaction. Later the oxidative work up means the oxidation takes place. But only aldehydes convert into carboxylic acids. The ketones will not convert into carboxylic acids is an important point.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




