
Why is a carbocation \[s{{p}^{2}}\] hybridized around the central carbon?
Answer
489.6k+ views
Hint: In valence bond theory, orbital hybridisation is the notion of combining atomic orbitals into new hybrid orbitals (with different energies, shapes, and other properties than the component atomic orbitals) appropriate for electron pairing to create chemical bonds. The VSEPR theory is a chemical model that uses the number of electron pairs surrounding a core atom to predict the geometry of individual molecules.
Complete answer:
A carbocation, according to the IUPAC, is any cation with an even number of electrons and a major amount of the positive charge on a carbon atom. Carbocation and carbonium ion were used interchangeably before Olah and associates discovered five-coordinate carbocations. Olah recommended that the carbonium ion be redefined as a carbocation with any form of three-center two-electron bonding, while the carbenium ion be defined as a carbocation with only two-center two-electron bonds and a three-coordinate positive carbon.
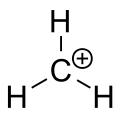
One s and two p atomic orbitals are mixed in the \[s{{p}^{2}}\] hybridization, which entails the promotion of one electron from the s orbital to one of the 2p atomic orbitals. When these atomic orbitals are combined, three new hybrid orbitals with the same energy level are created. The hybrid orbitals have a greater energy than the s orbital but a lower energy than the p orbitals, but they are closer to the p orbitals in terms of energy. The newly generated hybrid orbitals produce trigonal structures with a 120-degree molecular geometry.
The valence shell electron pair repulsion hypothesis posits that electron pairs surrounding a core atom form Platonic solids, which reduces electrostatic repulsion between bonded and lone pairs. Two electron pairs are organised linearly, three electron pairs in a trigonal plane, and four electron pairs in a tetrahedron. Because there are three bonding electron pairs around the carbon in \[^{+}C{{R}_{3}}\] , the bond angle \[\angle R-C-R\] is \[{{120}^{o}}\] . Because boron halides and alkyls are isoelectronic with \[^{+}C{{R}_{3}}\], \[B{{R}_{3}}\] is neutral in this situation.
Note:
Carbon will be \[s{{p}^{2}}\] hybridised in carbocation, and its form will be trigonal planar. There's also an empty p orbital, indicating that it's electron-poor. The valence shell of carbon has 6 electrons. It is an electron-deficient species, commonly known as an electrophile, as a result of this.
Complete answer:
A carbocation, according to the IUPAC, is any cation with an even number of electrons and a major amount of the positive charge on a carbon atom. Carbocation and carbonium ion were used interchangeably before Olah and associates discovered five-coordinate carbocations. Olah recommended that the carbonium ion be redefined as a carbocation with any form of three-center two-electron bonding, while the carbenium ion be defined as a carbocation with only two-center two-electron bonds and a three-coordinate positive carbon.

One s and two p atomic orbitals are mixed in the \[s{{p}^{2}}\] hybridization, which entails the promotion of one electron from the s orbital to one of the 2p atomic orbitals. When these atomic orbitals are combined, three new hybrid orbitals with the same energy level are created. The hybrid orbitals have a greater energy than the s orbital but a lower energy than the p orbitals, but they are closer to the p orbitals in terms of energy. The newly generated hybrid orbitals produce trigonal structures with a 120-degree molecular geometry.
The valence shell electron pair repulsion hypothesis posits that electron pairs surrounding a core atom form Platonic solids, which reduces electrostatic repulsion between bonded and lone pairs. Two electron pairs are organised linearly, three electron pairs in a trigonal plane, and four electron pairs in a tetrahedron. Because there are three bonding electron pairs around the carbon in \[^{+}C{{R}_{3}}\] , the bond angle \[\angle R-C-R\] is \[{{120}^{o}}\] . Because boron halides and alkyls are isoelectronic with \[^{+}C{{R}_{3}}\], \[B{{R}_{3}}\] is neutral in this situation.
Note:
Carbon will be \[s{{p}^{2}}\] hybridised in carbocation, and its form will be trigonal planar. There's also an empty p orbital, indicating that it's electron-poor. The valence shell of carbon has 6 electrons. It is an electron-deficient species, commonly known as an electrophile, as a result of this.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




