
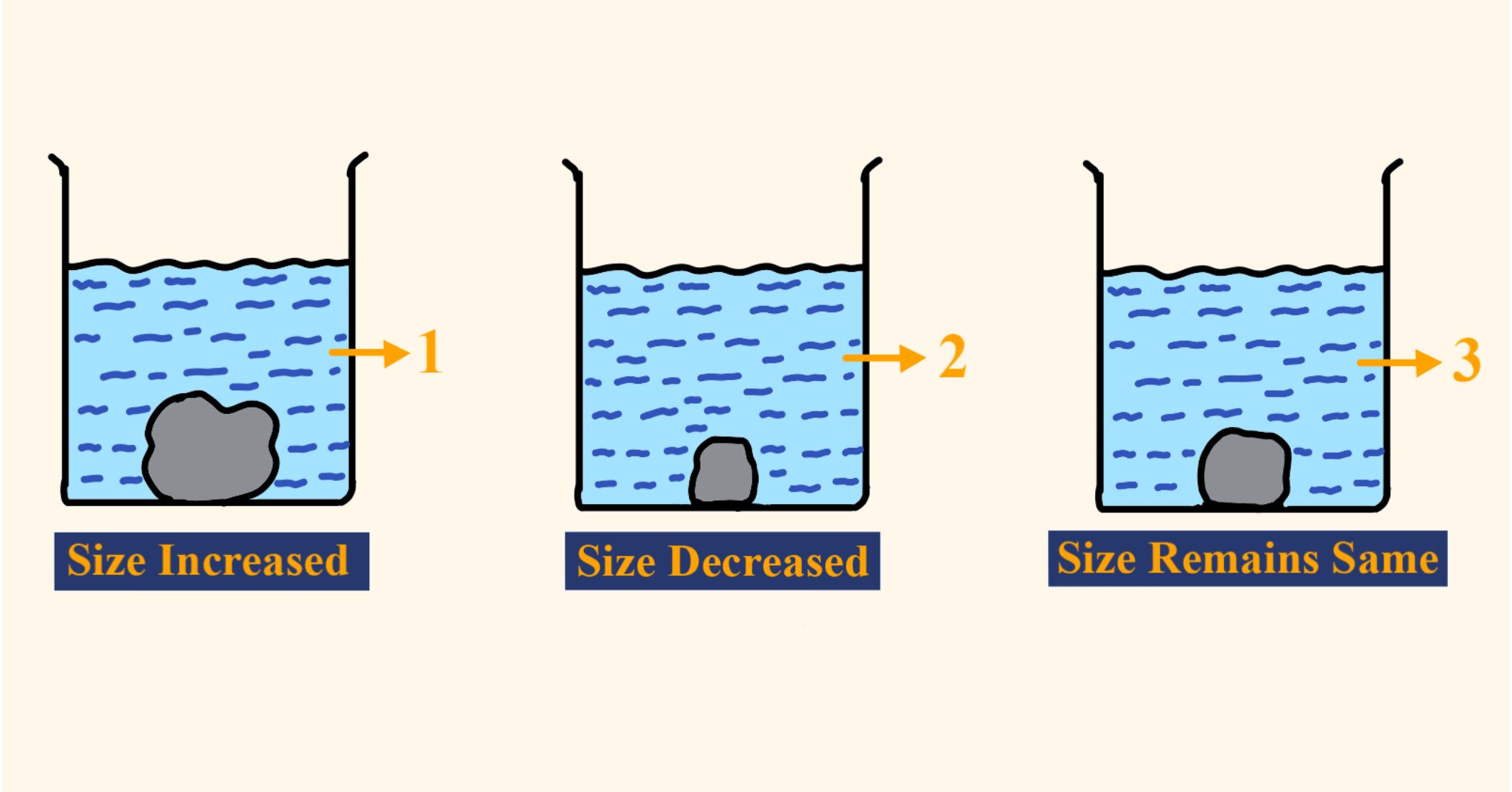
A candidate in order to study the process of osmosis has taken 3 potato cubes and put them in 3 different beakers containing 3 different solutions. After 24 hours, in the first beaker, the potato cube increased in size, in the second beaker the potato cube decreased in size, and in the third beaker, there was no change in the size of the potato cube. The following diagram shows the result of the same experiment:
(i)Give the technical terms of the solutions used in beakers 1,2, and 3.
(ii)In beaker 3 the size of the potato cube remains the same. Explain the reason in brief.
(iii)Write the specific feature of the cell sap of root hairs which helps in the absorption of water.
(iv)What is osmosis?
(v)How do a cell wall and a cell membrane differ in their permeability?

Answer
565.5k+ views
Hint: Osmosis is referred to as the movement of water (solvent) from its high concentration to low concentration i.e. in the downhill direction of the concentration gradient across a semipermeable or selectively permeable membrane like plasma membrane.
Complete answer:
Let us look at the parts of the question individually.
(i)The technical terms of the solutions used in beakers 1,2 and 3 are hypotonic, hypertonic, and isotonic solutions. The solution in beaker 1 is hypotonic as water moves from the solution inside the cells of the potato cube, resulting in it to swell. This is because the concentration of the solution is less than the concentration of the plasma of potato cells, and such a solution is known as a hypotonic solution. Beaker 2 has a hypertonic solution because its concentration is more than the concentration of potato cell plasma resulting in water moving out from the cells causing them to shrink. But there is no change in the size of the potato cube because the concentration of the solution is the same as the concentration of the plasma of cells (isotonic) and thus there is no net water movement in any direction.
(ii)The size of the potato cube in beaker 3 remains the same because the solution is an isotonic solution which means that the concentration of the solution is equal to the concentration of the plasma of the cells of the potato. This causes an equal amount of water to flow in and out of the cell thus resulting in no change in the size of the potato cube. Such cells are said to be in a flaccid state.
(iii)The special feature of the cell sap of root hairs is that it always remains hypertonic in nature to facilitate the easy absorption of water from the soil. This hypertonic condition is maintained by actively pumping minerals and ions inside the cell to keep the concentration of the cell sap high.
(iv)Osmosis is the movement of water across a selectively permeable membrane in response to a driving force. The rate of osmosis, as well as the net direction of osmosis, depends on the pressure gradient (pressure applied on a solution) and a concentration gradient. Water moves from a solution of low concentration (high water concentration) to a solution of high concentration (low water concentration.).
(v)The cell wall of a plant is freely permeable and does not provide resistance to the movement of substances in and out of the cell. Whereas the cell membrane is selectively permeable which means that it can select the substances that it wants to allow inside the cell according to the needs of the cell.
Note: -Imbibition is a special type of diffusion of water in which large amounts of water are absorbed by solids resulting in a sudden massive increase in their volume.
-Active transport is the process of transporting molecules against their concentration gradient by the utilization of energy.
-Plasmolysis occurs in a cell when it is placed in a hypertonic solution causing it to shrink away from its walls due to loss of water.
Complete answer:
Let us look at the parts of the question individually.
(i)The technical terms of the solutions used in beakers 1,2 and 3 are hypotonic, hypertonic, and isotonic solutions. The solution in beaker 1 is hypotonic as water moves from the solution inside the cells of the potato cube, resulting in it to swell. This is because the concentration of the solution is less than the concentration of the plasma of potato cells, and such a solution is known as a hypotonic solution. Beaker 2 has a hypertonic solution because its concentration is more than the concentration of potato cell plasma resulting in water moving out from the cells causing them to shrink. But there is no change in the size of the potato cube because the concentration of the solution is the same as the concentration of the plasma of cells (isotonic) and thus there is no net water movement in any direction.
(ii)The size of the potato cube in beaker 3 remains the same because the solution is an isotonic solution which means that the concentration of the solution is equal to the concentration of the plasma of the cells of the potato. This causes an equal amount of water to flow in and out of the cell thus resulting in no change in the size of the potato cube. Such cells are said to be in a flaccid state.
(iii)The special feature of the cell sap of root hairs is that it always remains hypertonic in nature to facilitate the easy absorption of water from the soil. This hypertonic condition is maintained by actively pumping minerals and ions inside the cell to keep the concentration of the cell sap high.
(iv)Osmosis is the movement of water across a selectively permeable membrane in response to a driving force. The rate of osmosis, as well as the net direction of osmosis, depends on the pressure gradient (pressure applied on a solution) and a concentration gradient. Water moves from a solution of low concentration (high water concentration) to a solution of high concentration (low water concentration.).
(v)The cell wall of a plant is freely permeable and does not provide resistance to the movement of substances in and out of the cell. Whereas the cell membrane is selectively permeable which means that it can select the substances that it wants to allow inside the cell according to the needs of the cell.
Note: -Imbibition is a special type of diffusion of water in which large amounts of water are absorbed by solids resulting in a sudden massive increase in their volume.
-Active transport is the process of transporting molecules against their concentration gradient by the utilization of energy.
-Plasmolysis occurs in a cell when it is placed in a hypertonic solution causing it to shrink away from its walls due to loss of water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




