
A. Calculate the formula unit mass of NaCl and ${\rm{CaC}}{{\rm{l}}_{\rm{2}}}$.
B.A sample of 45.8 g ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}$
contains how many moles of ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}$?
C. An element X has valency 3 while the element Y has valency 2. What is the chemical formula of the compound between X and Y?
Answer
558.9k+ views
Hint: We know that, formula unit mass of a substance is the sum of the atomic masses of all atoms in a formula unit of an ionic compound. This is similar to the calculation of molecular mass of compounds but the term ‘formula unit mass’ is used in case of ionic compounds only.
Complete step by step answer:
A) Here, both NaCl and ${\rm{CaC}}{{\rm{l}}_{\rm{2}}}$ are ionic compounds. So, we can calculate the formula unit mass. The calculation of formula unit mass is the same as molecular mass.
So, Formula unit mass of NaCl$ = 23 + 35.5 = 58.5\,{\rm{g}}\,{\rm{mo}}{{\rm{l}}^{ - 1}}$
Formula unit mass of ${\rm{CaC}}{{\rm{l}}_{\rm{2}}}$
$ = 40 + 2 \times 35.5 = 111\,{\rm{g}}\,{\rm{mo}}{{\rm{l}}^{ - 1}}$
B) Here, we have to calculate the moles of sulphuric acid $\left( {{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}} \right)$. The formula of number of moles is,
Number of moles=$\dfrac{{{\rm{Mass}}}}{{{\rm{Molar}}\,{\rm{mass}}}}$
The mass of sulphuric acid is given as 45.8 g and now we have to calculate the molar mass of ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}$.
Molar mass of ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}$
=$2 \times 1 + 32 + 4 \times 16 = 98\,{\rm{g}}\,{\rm{mo}}{{\rm{l}}^{ - 1}}$
Now, we have to use the above formula.
Number of moles of ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}$
=$\dfrac{{45.8\,{\rm{g}}}}{{98\,{\rm{g}}\,{\rm{mo}}{{\rm{l}}^{ - 1}}}} = 0.467\,{\rm{mol}}$
C) We know that the formula of a compound can be known if the valencies of the combining elements are known. We have to interchange the valencies of the combining elements to get the formula of the compound.
Given that valency of X is 3 and valency of Y is 2.
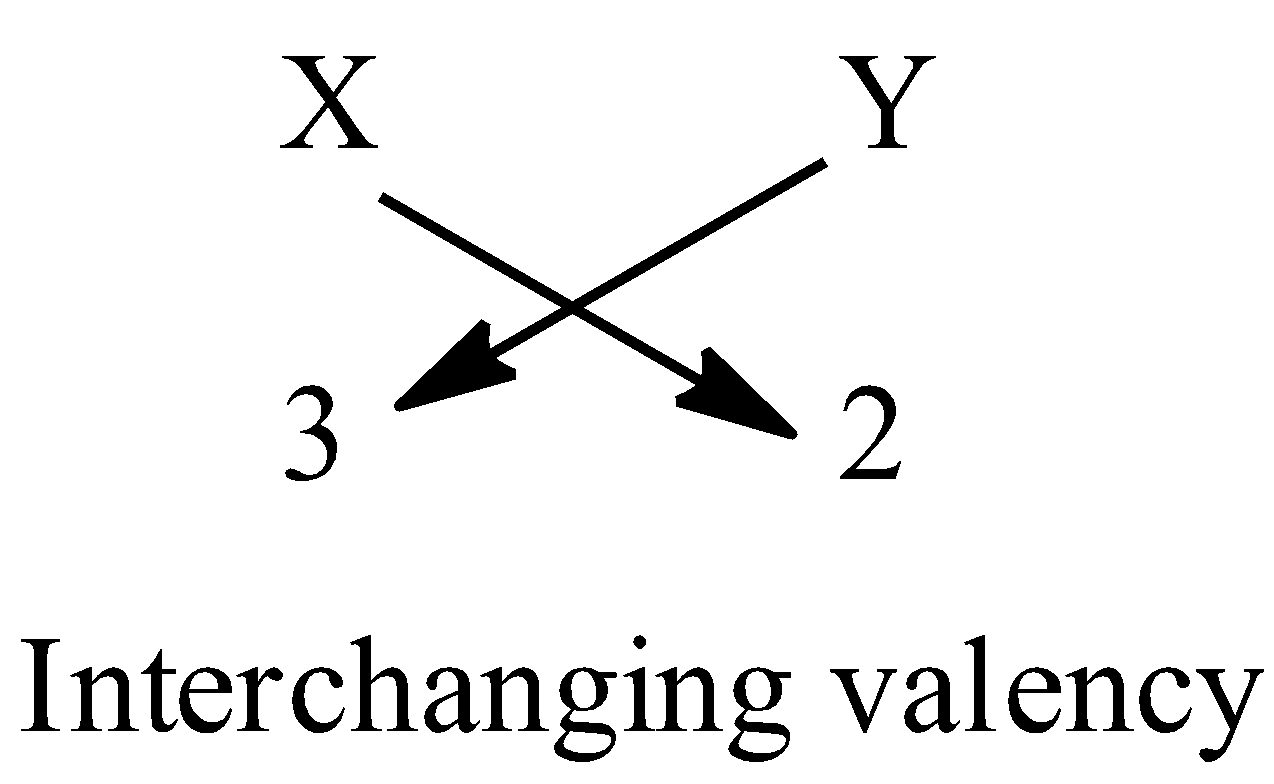
So, the formula of the compound XY is ${{\rm{X}}_{\rm{2}}}{{\rm{Y}}_{\rm{3}}}$.
Note: Always remember that calculation of molecular mass and formula unit mass is the same but the term formula unit mass is used in case of ionic compounds and not in case of covalent compounds.
Complete step by step answer:
A) Here, both NaCl and ${\rm{CaC}}{{\rm{l}}_{\rm{2}}}$ are ionic compounds. So, we can calculate the formula unit mass. The calculation of formula unit mass is the same as molecular mass.
So, Formula unit mass of NaCl$ = 23 + 35.5 = 58.5\,{\rm{g}}\,{\rm{mo}}{{\rm{l}}^{ - 1}}$
Formula unit mass of ${\rm{CaC}}{{\rm{l}}_{\rm{2}}}$
$ = 40 + 2 \times 35.5 = 111\,{\rm{g}}\,{\rm{mo}}{{\rm{l}}^{ - 1}}$
B) Here, we have to calculate the moles of sulphuric acid $\left( {{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}} \right)$. The formula of number of moles is,
Number of moles=$\dfrac{{{\rm{Mass}}}}{{{\rm{Molar}}\,{\rm{mass}}}}$
The mass of sulphuric acid is given as 45.8 g and now we have to calculate the molar mass of ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}$.
Molar mass of ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}$
=$2 \times 1 + 32 + 4 \times 16 = 98\,{\rm{g}}\,{\rm{mo}}{{\rm{l}}^{ - 1}}$
Now, we have to use the above formula.
Number of moles of ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}$
=$\dfrac{{45.8\,{\rm{g}}}}{{98\,{\rm{g}}\,{\rm{mo}}{{\rm{l}}^{ - 1}}}} = 0.467\,{\rm{mol}}$
C) We know that the formula of a compound can be known if the valencies of the combining elements are known. We have to interchange the valencies of the combining elements to get the formula of the compound.
Given that valency of X is 3 and valency of Y is 2.

So, the formula of the compound XY is ${{\rm{X}}_{\rm{2}}}{{\rm{Y}}_{\rm{3}}}$.
Note: Always remember that calculation of molecular mass and formula unit mass is the same but the term formula unit mass is used in case of ionic compounds and not in case of covalent compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




