
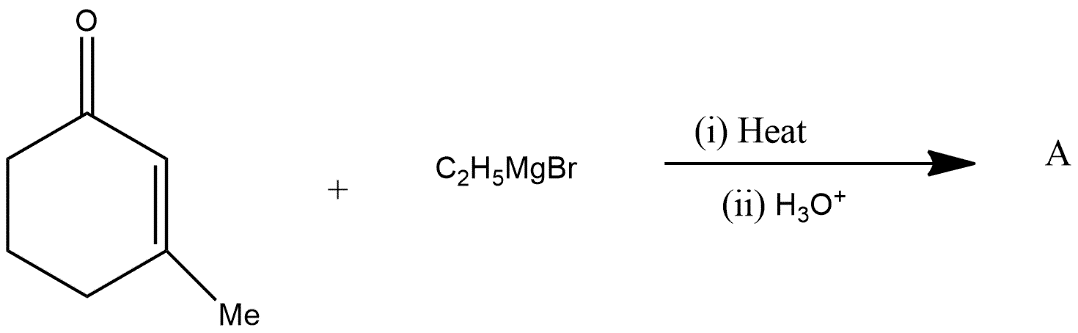
A.

B.
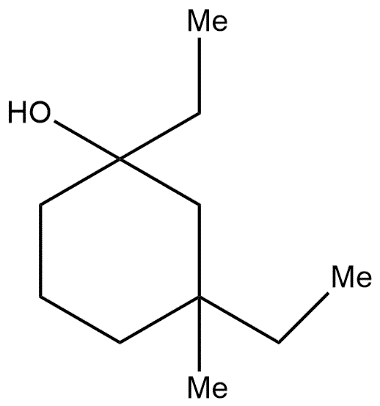
C.

D. Both A and B
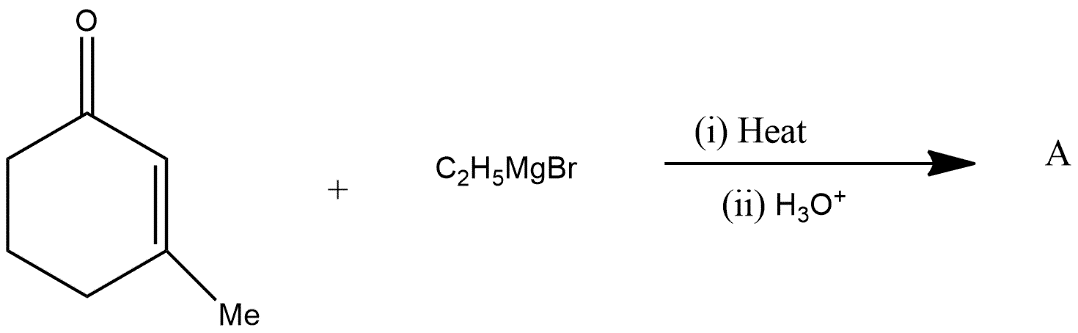



Answer
576.6k+ views
Hint:. Grignard reaction is an organometallic chemical reaction in which alkyl, vinyl, allyl or aryl magnesium bromide which is known by Grignard reagent is added to a carbonyl group in ketone or aldehydes. This reaction plays an important role in making C-C bonds.
Complete step by step answer:
The reaction given above is generally an organometallic reaction which provides a Grignard reagent. The mechanism of reaction involved, carbon attached to magnesium functions acts as a nucleophile which attacks on the electrophilic carbon atom which is present within the polar bond of a carbonyl group. The addition of the Grignard reagent to the carbonyl group form an intermediate state showing six membered ring and the mechanism is shown as follows:
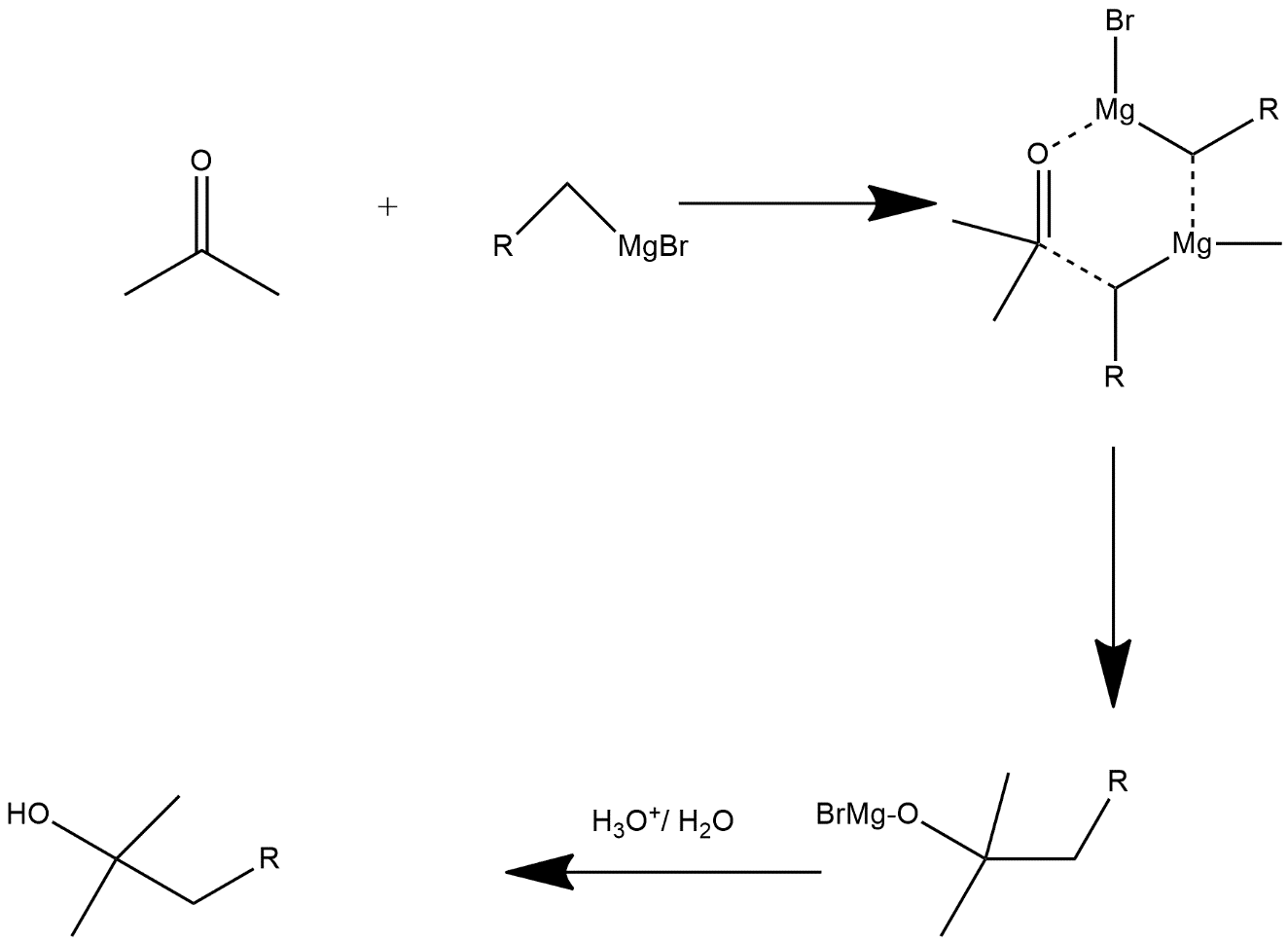
As in the first step it forms an intermediate state of 6-membered ring it is not actually a six-membered ring it is generally the representation of formation of bonds and after that we get the stable structure which further reacts with water molecules and forms OH group compound. Hence from the above mechanism we come to know that in the product there is presence of OH i.e. alcoholic group is there so from all options the correct answer is “Option A”.
Note: Pure Grignard reagents are very much reactive solids. It can be normally handled as solutions in solvent like diethyl ether or tetrahydrofuran which is very much stable. In these type of medium Grignard reagent is present as a complex with the magnesium atom connected to the two ether or oxygen by coordinate bond.
Complete step by step answer:
The reaction given above is generally an organometallic reaction which provides a Grignard reagent. The mechanism of reaction involved, carbon attached to magnesium functions acts as a nucleophile which attacks on the electrophilic carbon atom which is present within the polar bond of a carbonyl group. The addition of the Grignard reagent to the carbonyl group form an intermediate state showing six membered ring and the mechanism is shown as follows:

As in the first step it forms an intermediate state of 6-membered ring it is not actually a six-membered ring it is generally the representation of formation of bonds and after that we get the stable structure which further reacts with water molecules and forms OH group compound. Hence from the above mechanism we come to know that in the product there is presence of OH i.e. alcoholic group is there so from all options the correct answer is “Option A”.
Note: Pure Grignard reagents are very much reactive solids. It can be normally handled as solutions in solvent like diethyl ether or tetrahydrofuran which is very much stable. In these type of medium Grignard reagent is present as a complex with the magnesium atom connected to the two ether or oxygen by coordinate bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




