
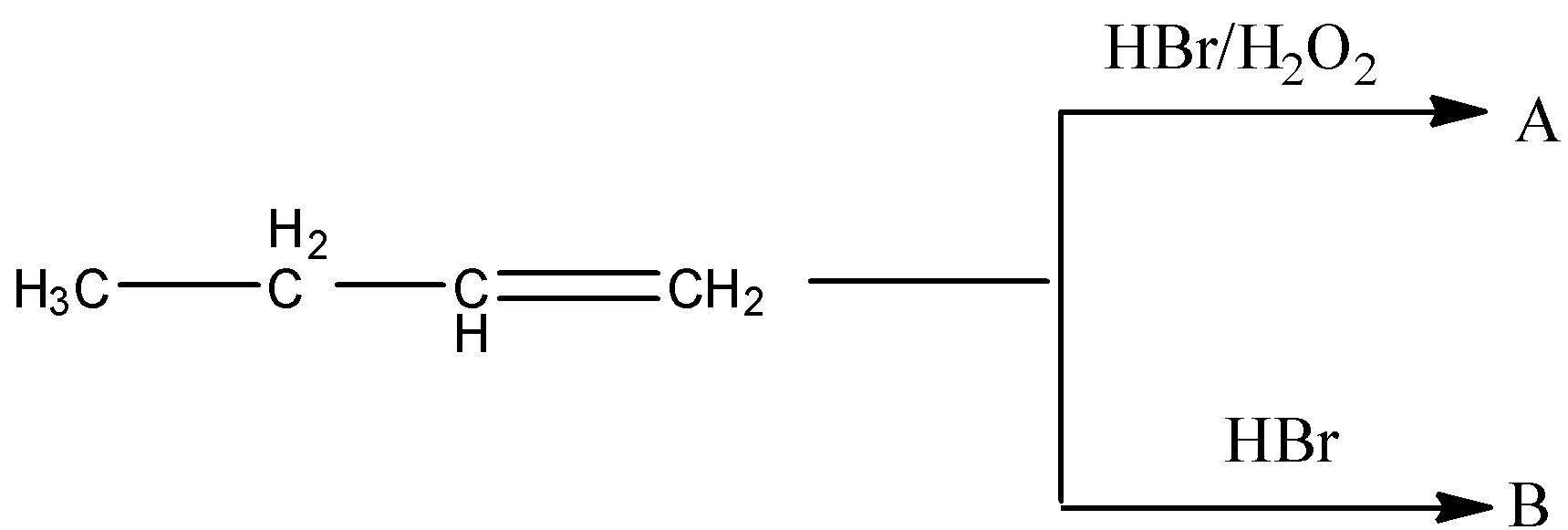
A and B are related as
(1) Chain isomers
(2) Position isomers
(3) Functional isomers
(4) Tautomers

Answer
559.2k+ views
Hint: We know that isomerism is the phenomenon in which two or more compounds have the same chemical formula but different structures. The compounds showing isomerism are known as isomers. There are different types of isomers, such as, position isomers, tautomers, chain isomers etc.
Complete step by step answer:
Let’s first discuss chain isomers, functional isomers, position isomer and tautomer in detail.
1)Chain isomerism is a phenomenon in which isomers differ in the carbon chain. For example, ${{\rm{C}}_{\rm{4}}}{{\rm{H}}_{{\rm{10}}}}$ represents two chain isomers namely butane and 2-methylpropane.
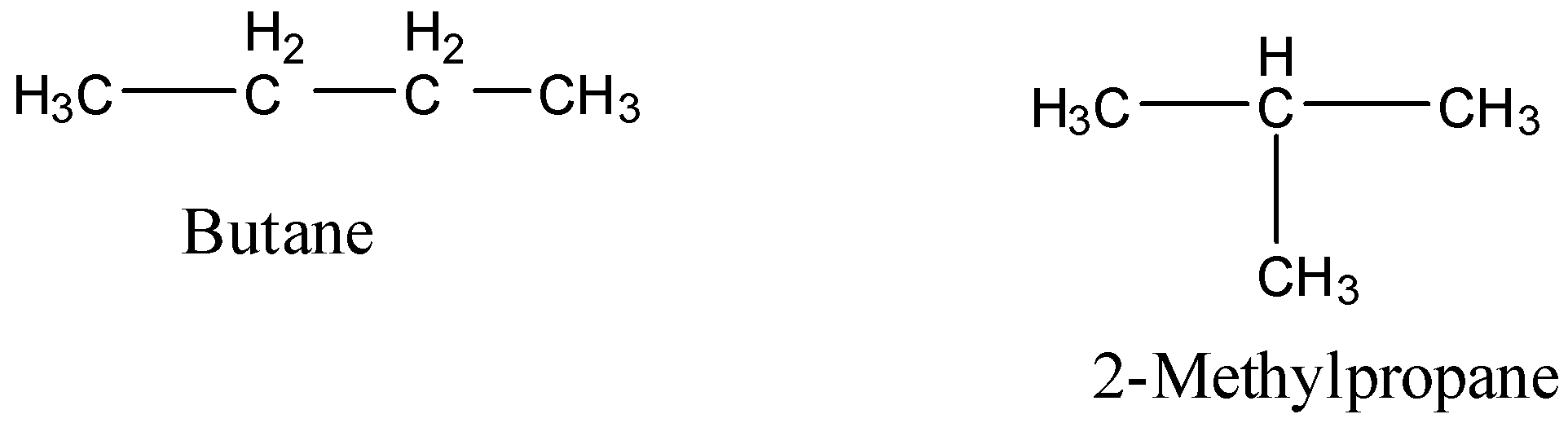
2)Functional isomers are the isomers that differ with respect to the nature of functional groups. So, both the isomers belong to different families. For example, ${{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{6}}}{\rm{O}}$ has two functional isomers.

3)Position isomers are the isomers which differ with respect to the position of multiple bonds (double or triple), substituents or functional groups. For example, ${{\rm{C}}_{\rm{3}}}{{\rm{H}}_{\rm{7}}}{\rm{OH}}$ has two position isomers.
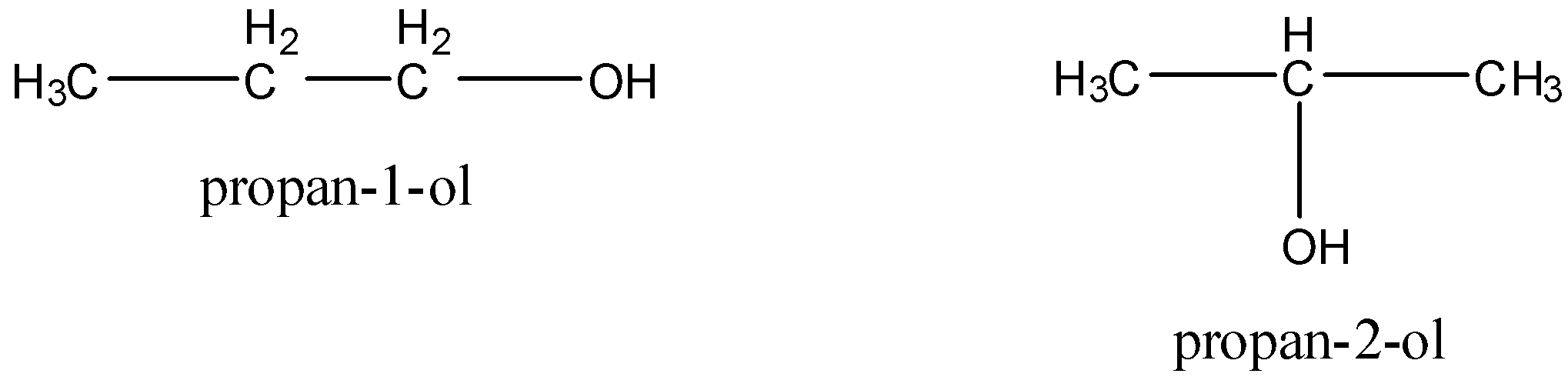
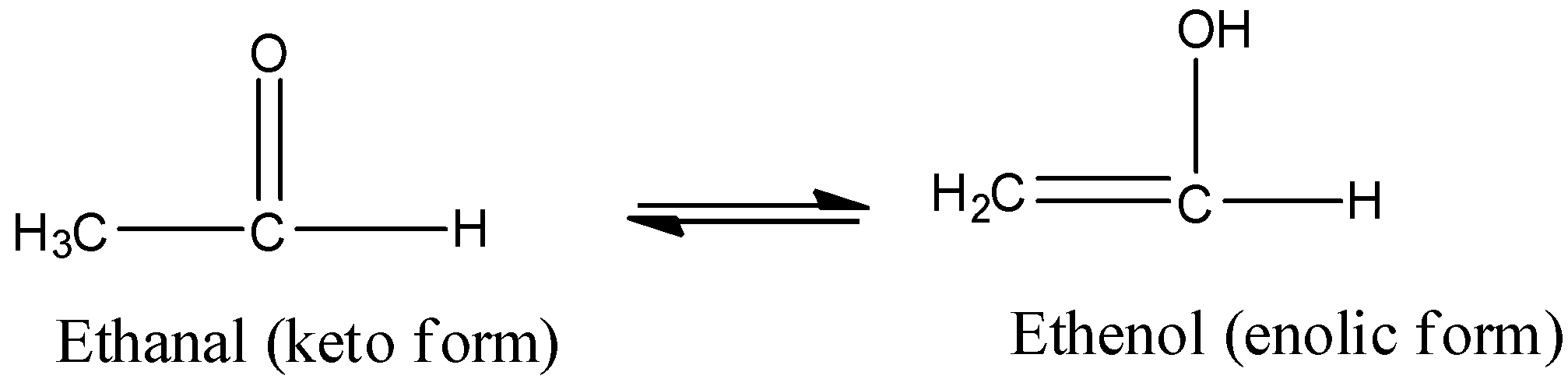
4)Tautomers are actually functional isomers which exist simultaneously and also in dynamic equilibrium. The isomerism in this case is termed as tautomerism. It is of different types but the most common among them is the keto-enol tautomerism. This isomerism arises due to 1,3 migration of the hydrogen atom from carbon to oxygen atom and vice versa. For example,
Now, we have to write the products of two reactions of butane.
First reaction is butene reacts with HBr in presence of ${{\rm{H}}_{\rm{2}}}{{\rm{O}}_{\rm{2}}}$. This is a substitution reaction. So, the product of the reaction is,
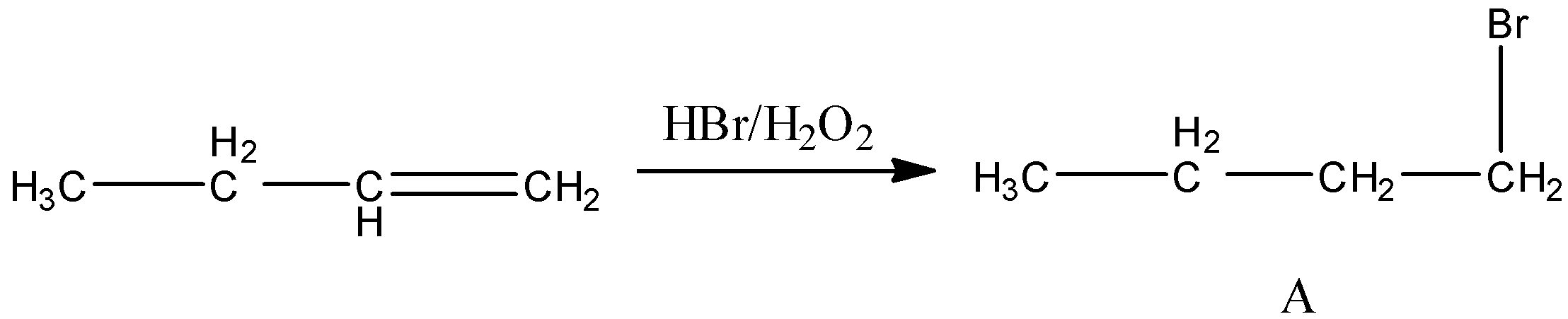
Second reaction is butane reacts with HBr. This reaction is a Markovnikov reaction, that means bromine is bonded to that carbon atom of the double bond in which the lowest number of hydrogen atoms is bonded.

Now, we identify the type of isomer A and B is. Only difference between A and B is the position of the bromine group. So, A and B are position isomers.
So, the correct answer is Option 3.
Note: Always remember that both alkenes and alkynes can show chain as well as position isomerism for the same molecular formula. And alkanes can show only chain isomerism and not any position isomerism since all carbon bonds are single bonds.
Complete step by step answer:
Let’s first discuss chain isomers, functional isomers, position isomer and tautomer in detail.
1)Chain isomerism is a phenomenon in which isomers differ in the carbon chain. For example, ${{\rm{C}}_{\rm{4}}}{{\rm{H}}_{{\rm{10}}}}$ represents two chain isomers namely butane and 2-methylpropane.
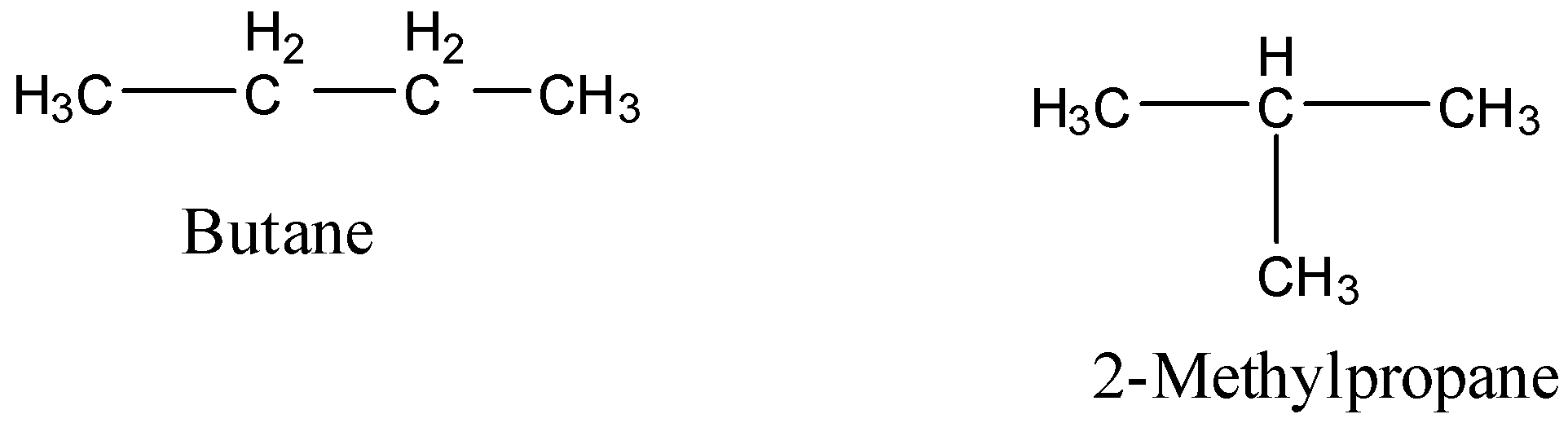
2)Functional isomers are the isomers that differ with respect to the nature of functional groups. So, both the isomers belong to different families. For example, ${{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{6}}}{\rm{O}}$ has two functional isomers.

3)Position isomers are the isomers which differ with respect to the position of multiple bonds (double or triple), substituents or functional groups. For example, ${{\rm{C}}_{\rm{3}}}{{\rm{H}}_{\rm{7}}}{\rm{OH}}$ has two position isomers.
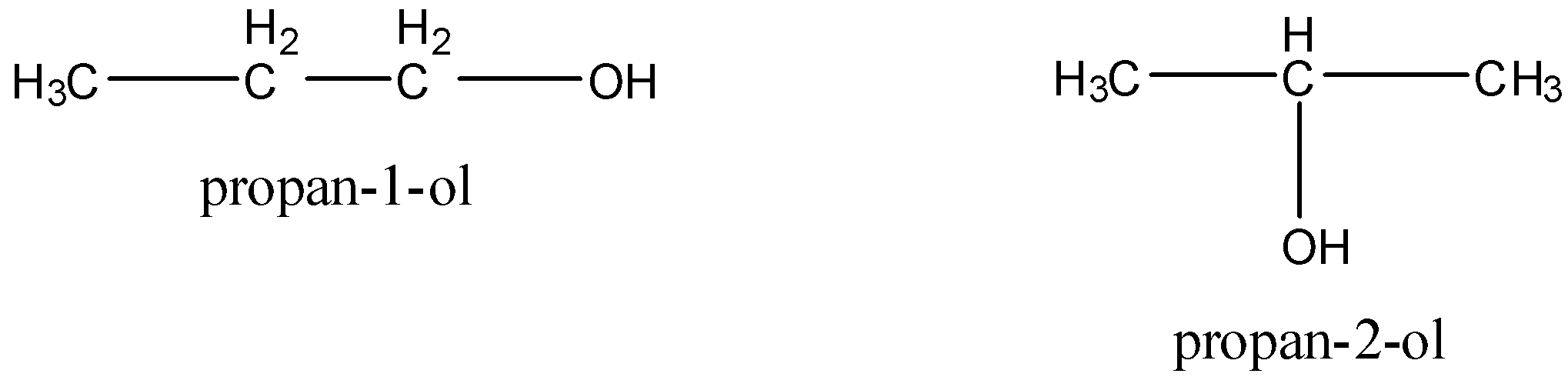
4)Tautomers are actually functional isomers which exist simultaneously and also in dynamic equilibrium. The isomerism in this case is termed as tautomerism. It is of different types but the most common among them is the keto-enol tautomerism. This isomerism arises due to 1,3 migration of the hydrogen atom from carbon to oxygen atom and vice versa. For example,

Now, we have to write the products of two reactions of butane.
First reaction is butene reacts with HBr in presence of ${{\rm{H}}_{\rm{2}}}{{\rm{O}}_{\rm{2}}}$. This is a substitution reaction. So, the product of the reaction is,

Second reaction is butane reacts with HBr. This reaction is a Markovnikov reaction, that means bromine is bonded to that carbon atom of the double bond in which the lowest number of hydrogen atoms is bonded.

Now, we identify the type of isomer A and B is. Only difference between A and B is the position of the bromine group. So, A and B are position isomers.
So, the correct answer is Option 3.
Note: Always remember that both alkenes and alkynes can show chain as well as position isomerism for the same molecular formula. And alkanes can show only chain isomerism and not any position isomerism since all carbon bonds are single bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




