
A 9V battery is connected to a bulb whose resistance is $3000\;\Omega$. How many electrons leave the battery per minute?
Answer
540.3k+ views
Hint: We know that a battery establishes a potential difference across the ends of the circuit enabling current to flow through it. In such a case, first determine the current across the circuit supplied by the battery. Then, we know that the current is nothing but charge flowing through the circuit in unit time. To this end, determine the charge through the circuit, and by considering the charge possessed by a single electron, deduce the number of electrons leaving the battery in one minute. Pay caution to the unit of time for all calculations.
Formula Used:
Current $I = \dfrac{V}{R}$
Charge $Q = I \times t$
Complete answer:
We are given that $V =9\;V$ and $R = 3000\;\Omega$.

Therefore, the current flowing in the bulb due to the battery can be given by:
$I = \dfrac{V}{R} = \dfrac{9}{3000} = 3 \times 10^{-3}\;A$
Now, we know that current is equivalent to the charge flowing out the battery in unit time, i.e.,
$I = \dfrac{Q}{t}$
Therefore, the charge flowing out the battery can be given as:
$Q = I \times t$
Taking $t = 1\;min = 60\;s$ and $I = 3 \times 10^{-3}\;A$ the above equation becomes:
$Q = 3 \times 10^{-3} \times 60 = 180 \times 10^{-3}\;C$
Now, we know that a single electron carries a charge of $1.6 \times 10^{-19}\;C$
Therefore, the number of electrons carrying a charge of $Q= 180 \times 10^{-3}\;C$ will be:
$n = \dfrac{180 \times 10^{-3}}{1.6 \times 10^{-19}} = 112.5 \times 10^{-3+19} = 112.5 \times 10^{16} \approx 113 \times 10^{16}$
Thus, about $113 \times 10^{16}$ electrons leave the battery per minute.
Note:
It is important to understand the distinction between electron flow and conventional current flow. Electrons flow from the negative terminal of the battery to the positive terminal and constitute the electron current whereas positive charges move in an opposite direction and constitute the conventional current.
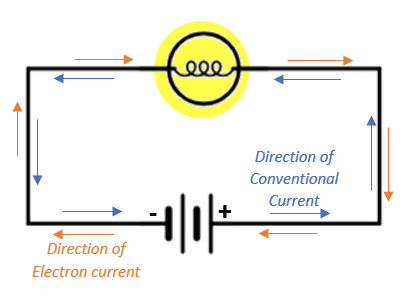
Keep in mind that the electric current in a circuit arises only from the flow of electrons and not from the flow of positively charged particles. Thus, conventional current is defined as if the movement of positive charge carriers constitute electric current flow when it is in fact not the case.
Formula Used:
Current $I = \dfrac{V}{R}$
Charge $Q = I \times t$
Complete answer:
We are given that $V =9\;V$ and $R = 3000\;\Omega$.

Therefore, the current flowing in the bulb due to the battery can be given by:
$I = \dfrac{V}{R} = \dfrac{9}{3000} = 3 \times 10^{-3}\;A$
Now, we know that current is equivalent to the charge flowing out the battery in unit time, i.e.,
$I = \dfrac{Q}{t}$
Therefore, the charge flowing out the battery can be given as:
$Q = I \times t$
Taking $t = 1\;min = 60\;s$ and $I = 3 \times 10^{-3}\;A$ the above equation becomes:
$Q = 3 \times 10^{-3} \times 60 = 180 \times 10^{-3}\;C$
Now, we know that a single electron carries a charge of $1.6 \times 10^{-19}\;C$
Therefore, the number of electrons carrying a charge of $Q= 180 \times 10^{-3}\;C$ will be:
$n = \dfrac{180 \times 10^{-3}}{1.6 \times 10^{-19}} = 112.5 \times 10^{-3+19} = 112.5 \times 10^{16} \approx 113 \times 10^{16}$
Thus, about $113 \times 10^{16}$ electrons leave the battery per minute.
Note:
It is important to understand the distinction between electron flow and conventional current flow. Electrons flow from the negative terminal of the battery to the positive terminal and constitute the electron current whereas positive charges move in an opposite direction and constitute the conventional current.

Keep in mind that the electric current in a circuit arises only from the flow of electrons and not from the flow of positively charged particles. Thus, conventional current is defined as if the movement of positive charge carriers constitute electric current flow when it is in fact not the case.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




