
A 3-d transition metal cation ${x^{3 + }}$ has the magnetic moment $\sqrt {35} $ BM. What is the atomic number of metal x?
(A) 24
(B) 25
(C) 26
(D) 27
Answer
591k+ views
Hint: The two important clues given in the question are one is that the element has 3-d orbital and secondly the value of magnetic moment value is given. We should be aware of magnetic moments.
Complete step by step solution:
The magnetic moment formula is given by:
\[\mu = \sqrt {n(n + 2)} \]
Where, $\mu $= magnetic moment
n = number of unpaired electrons
According to question, $\mu = \sqrt {35} $
So, 35 = n (n+2)
n = 5
As mentioned above n is the number of unpaired electrons, Thus, the element has 5 unpaired electrons.
The 3d transition metals are the transitional method that has the 3d orbital as an outermost shell. The elements of 3d series are scandium, Titanium, Vanadium, Chromium, manganese, iron, cobalt, nickel, copper and zinc.
Option A is 24. The atomic number of chromium is 24. The electronic configuration of chromium is $[Ar]3{d^5}4{s^1}$.
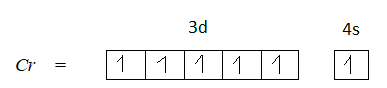
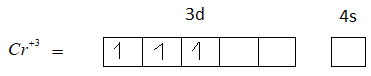
The element in its +3 oxidation state should contain 5 unpaired electrons, but $C{r^{ + 3}}$ has only 3 unpaired electrons. Therefore, option A is not the correct answer.
Option B is 25. The atomic number of manganese is 25. The electronic configuration of manganese is$[Ar]3{d^5}4{s^2}$.
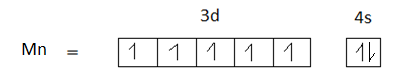
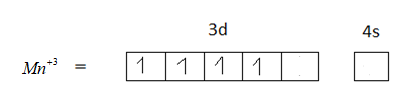
The element in its +3 oxidation state should contain 5 unpaired electrons, but $M{n^{ + 3}}$ has 4 unpaired electrons. Therefore, option B is not the correct answer.
Option C is 26. The atomic number of Iron is 26. The electronic configuration of iron is $[Ar]3{d^7}4{s^2}$.
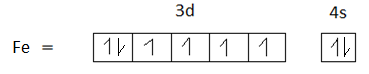
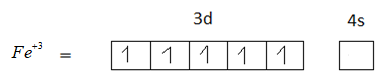
$F{e^{ + 3}}$ has 5 unpaired electrons in its +3 oxidation state. Therefore, option C is the correct answer.
Option D is 27. The atomic number of cobalt is 27.the electronic configuration of cobalt is $[Ar]3{d^7}4{s^2}$.
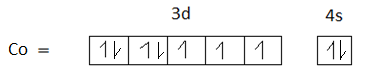
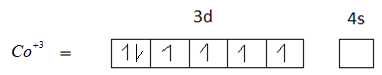
$C{o^{ + 3}}$ has 6 unpaired electrons. Therefore, Option D is not the correct answer.
Thus, the correct answer is option C.
Note: The whole answer revolves around electronic configuration and magnetic moment, so it’s very important to know the atomic number of elements in the periodic table. Always make sure that you carefully read the question and extract the given information. The elements of d-block readily lose and form half filled orbits that make them more reactive.
Complete step by step solution:
The magnetic moment formula is given by:
\[\mu = \sqrt {n(n + 2)} \]
Where, $\mu $= magnetic moment
n = number of unpaired electrons
According to question, $\mu = \sqrt {35} $
So, 35 = n (n+2)
n = 5
As mentioned above n is the number of unpaired electrons, Thus, the element has 5 unpaired electrons.
The 3d transition metals are the transitional method that has the 3d orbital as an outermost shell. The elements of 3d series are scandium, Titanium, Vanadium, Chromium, manganese, iron, cobalt, nickel, copper and zinc.
Option A is 24. The atomic number of chromium is 24. The electronic configuration of chromium is $[Ar]3{d^5}4{s^1}$.


The element in its +3 oxidation state should contain 5 unpaired electrons, but $C{r^{ + 3}}$ has only 3 unpaired electrons. Therefore, option A is not the correct answer.
Option B is 25. The atomic number of manganese is 25. The electronic configuration of manganese is$[Ar]3{d^5}4{s^2}$.


The element in its +3 oxidation state should contain 5 unpaired electrons, but $M{n^{ + 3}}$ has 4 unpaired electrons. Therefore, option B is not the correct answer.
Option C is 26. The atomic number of Iron is 26. The electronic configuration of iron is $[Ar]3{d^7}4{s^2}$.


$F{e^{ + 3}}$ has 5 unpaired electrons in its +3 oxidation state. Therefore, option C is the correct answer.
Option D is 27. The atomic number of cobalt is 27.the electronic configuration of cobalt is $[Ar]3{d^7}4{s^2}$.


$C{o^{ + 3}}$ has 6 unpaired electrons. Therefore, Option D is not the correct answer.
Thus, the correct answer is option C.
Note: The whole answer revolves around electronic configuration and magnetic moment, so it’s very important to know the atomic number of elements in the periodic table. Always make sure that you carefully read the question and extract the given information. The elements of d-block readily lose and form half filled orbits that make them more reactive.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Coming together federation is practiced in A India class 12 social science CBSE

Write the formula to find the shortest distance between class 12 maths CBSE




