
A 12.5eV electron beam is used to excite a gaseous hydrogen atom at room temperature. Determine the wavelengths and the corresponding series of the lines emitted.
Answer
569.4k+ views
Hint: Firstly, you could go for finding the energy after excitation of the hydrogen and thus determine its final excited state. After that you could find all possible transitions. Then you could find the energy difference of these levels and also the wavelength of the radiation emitted.
Formula used:
Energy of nth energy level,
${{E}_{n}}=\dfrac{-13.6eV}{{{n}^{2}}}$
Wavelength,
$\lambda =\dfrac{hc}{E}$
Complete answer:
We are given that the gaseous hydrogen atom is excited using a 12.5eV electron beam at room temperature. Then, its energy after excitation would be,
$E=-13.6eV+12.5eV=-1.1eV$
We know that energy of the nth energy level is given by,
${{E}_{n}}=\dfrac{-13.6eV}{{{n}^{2}}}$
$\Rightarrow \dfrac{-13.6}{{{n}^{2}}}=-1.1eV$
$\Rightarrow n=3.51\langle 4$
$\therefore n\approx 3$
So we found the final excited state to be n = 3.
So the possible transitions would be,
(a) $n=3\to n=2$
(b) $n=3\to n=1$
(c) $n=2\to n=1$
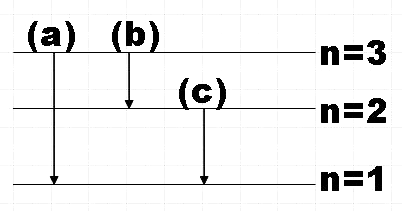
For the transition from$n=3\to n=2$,
$\Delta E={{E}_{3}}-{{E}_{2}}=\dfrac{-13.6}{{{3}^{2}}}-\left( \dfrac{-13.6}{{{2}^{2}}} \right)$
$\Rightarrow \Delta E=-1.51+3.4$
$\therefore \Delta E=1.89eV$
Corresponding wavelength,
$\lambda =\dfrac{hc}{E}$
$\Rightarrow \lambda =\dfrac{6.6\times {{10}^{-34}}\times 3\times {{10}^{8}}}{1.89\times 1.6\times {{10}^{-19}}}$
$\therefore \lambda =6547.6A{}^\circ $
So we found that this belongs to the Balmer series.
For the transition from$n=3\to n=1$,
$\Delta E={{E}_{3}}-{{E}_{1}}=\dfrac{-13.6}{{{3}^{2}}}-\left( \dfrac{-13.6}{{{1}^{2}}} \right)$
$\Rightarrow \Delta E=-1.51+13.6$
$\therefore \Delta E=12.09eV$
Corresponding wavelength,
$\lambda =\dfrac{hc}{E}$
$\Rightarrow \lambda =\dfrac{6.6\times {{10}^{-34}}\times 3\times {{10}^{8}}}{12.09\times 1.6\times {{10}^{-19}}}$
$\therefore \lambda =1023A{}^\circ $
So, we found that this belongs to the Lyman series.
For the transition from$n=2\to n=1$,
$\Delta E={{E}_{3}}-{{E}_{1}}=\dfrac{-13.6}{{{2}^{2}}}-\left( \dfrac{-13.6}{{{1}^{2}}} \right)$
$\Rightarrow \Delta E=-3.4+13.6$
$\therefore \Delta E=10.2eV$
Corresponding wavelength,
$\lambda =\dfrac{hc}{E}$
$\Rightarrow \lambda =\dfrac{6.6\times {{10}^{-34}}\times 3\times {{10}^{8}}}{10.2\times 1.6\times {{10}^{-19}}}$
$\therefore \lambda =1213A{}^\circ $
We found that this belongs to the Lyman series.
Note:
We should understand that the Hydrogen’s emission spectrum is basically divided into a number of spectral series. Also we know that they are observed as the result of electron transitions in various energy levels. We could also classify these series as Lyman series, Balmer series, Paschen series, Brackett series and Pfund series.
Formula used:
Energy of nth energy level,
${{E}_{n}}=\dfrac{-13.6eV}{{{n}^{2}}}$
Wavelength,
$\lambda =\dfrac{hc}{E}$
Complete answer:
We are given that the gaseous hydrogen atom is excited using a 12.5eV electron beam at room temperature. Then, its energy after excitation would be,
$E=-13.6eV+12.5eV=-1.1eV$
We know that energy of the nth energy level is given by,
${{E}_{n}}=\dfrac{-13.6eV}{{{n}^{2}}}$
$\Rightarrow \dfrac{-13.6}{{{n}^{2}}}=-1.1eV$
$\Rightarrow n=3.51\langle 4$
$\therefore n\approx 3$
So we found the final excited state to be n = 3.
So the possible transitions would be,
(a) $n=3\to n=2$
(b) $n=3\to n=1$
(c) $n=2\to n=1$

For the transition from$n=3\to n=2$,
$\Delta E={{E}_{3}}-{{E}_{2}}=\dfrac{-13.6}{{{3}^{2}}}-\left( \dfrac{-13.6}{{{2}^{2}}} \right)$
$\Rightarrow \Delta E=-1.51+3.4$
$\therefore \Delta E=1.89eV$
Corresponding wavelength,
$\lambda =\dfrac{hc}{E}$
$\Rightarrow \lambda =\dfrac{6.6\times {{10}^{-34}}\times 3\times {{10}^{8}}}{1.89\times 1.6\times {{10}^{-19}}}$
$\therefore \lambda =6547.6A{}^\circ $
So we found that this belongs to the Balmer series.
For the transition from$n=3\to n=1$,
$\Delta E={{E}_{3}}-{{E}_{1}}=\dfrac{-13.6}{{{3}^{2}}}-\left( \dfrac{-13.6}{{{1}^{2}}} \right)$
$\Rightarrow \Delta E=-1.51+13.6$
$\therefore \Delta E=12.09eV$
Corresponding wavelength,
$\lambda =\dfrac{hc}{E}$
$\Rightarrow \lambda =\dfrac{6.6\times {{10}^{-34}}\times 3\times {{10}^{8}}}{12.09\times 1.6\times {{10}^{-19}}}$
$\therefore \lambda =1023A{}^\circ $
So, we found that this belongs to the Lyman series.
For the transition from$n=2\to n=1$,
$\Delta E={{E}_{3}}-{{E}_{1}}=\dfrac{-13.6}{{{2}^{2}}}-\left( \dfrac{-13.6}{{{1}^{2}}} \right)$
$\Rightarrow \Delta E=-3.4+13.6$
$\therefore \Delta E=10.2eV$
Corresponding wavelength,
$\lambda =\dfrac{hc}{E}$
$\Rightarrow \lambda =\dfrac{6.6\times {{10}^{-34}}\times 3\times {{10}^{8}}}{10.2\times 1.6\times {{10}^{-19}}}$
$\therefore \lambda =1213A{}^\circ $
We found that this belongs to the Lyman series.
Note:
We should understand that the Hydrogen’s emission spectrum is basically divided into a number of spectral series. Also we know that they are observed as the result of electron transitions in various energy levels. We could also classify these series as Lyman series, Balmer series, Paschen series, Brackett series and Pfund series.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




