
3-phenylpropanoic acid is the IUPAC name of which of the compound?
A.Cinnamic acid
B. Mendalaeic acid
C.Pyruvic acid
D.Citric acid
Answer
579k+ views
Hint: Compound with the name 3-phenylpropanoic acid contains a benzene ring and it is a crystalline compound. It is used as a flavoring agent.
Complete step by step answer:
Let us look at the molecular formula of each of the options given to us:
Cinnamic acid is an organic compound. The molecular structure of cinnamic acid is as follow:
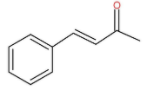
The IUPAC name of cinnamic acid is (2E)-3-Phenylprop-2-enoic acid. It also occurs in many plants naturally. It is used as an intermediate for biosynthesis and is obtained from oils of cinnamon. It is also found in Shea butter. It is also used in Indigo production and other pharmaceuticals. It is mainly used to produce methyl cinnamate, ethyl cinnamate and benzyl cinnamate for the use of perfumes. It also acts as a sweetener.
The molecular structure of Mandelic acid Is as follow:
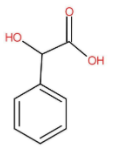
The IUPAC name of mandelic acid is hydroxyl(phenyl)acetic acid.
The molecular formula of pyruvic acid is as follow:
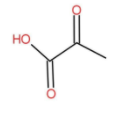
The IUPAC name is 2-Oxopropanoic acid. It is an Alpha keto acid and has no benzene ring.
The structure of citric acid is as follow:
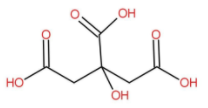
The IUPAC name is 2-hydroxypropane-1,2,3-tricarboxylic acid. Citric acid naturally occurs in various fruits and vegetables. Lemons have considerable high concentration of citric acid. It is also contained in grape food, oranges. Mainly citric acid is used as a flavoring agent and as a preservative in food and beverages specially used in soft drinks and candies.
Hence, the correct option is option A.
Note:
There are various methods of preparation of cinnamic acid. It was first prepared by base catalysed condensation with benzaldehyde and acetone chloride followed by the hydrolysis. It can also be prepared using Knovengel condensation reaction, using benzaldehyde and malonic acid along with some weak base then acid catalysed decarboxylation. It can also be prepared with the help of oxidation of cinnamaldehyde.
Complete step by step answer:
Let us look at the molecular formula of each of the options given to us:
Cinnamic acid is an organic compound. The molecular structure of cinnamic acid is as follow:

The IUPAC name of cinnamic acid is (2E)-3-Phenylprop-2-enoic acid. It also occurs in many plants naturally. It is used as an intermediate for biosynthesis and is obtained from oils of cinnamon. It is also found in Shea butter. It is also used in Indigo production and other pharmaceuticals. It is mainly used to produce methyl cinnamate, ethyl cinnamate and benzyl cinnamate for the use of perfumes. It also acts as a sweetener.
The molecular structure of Mandelic acid Is as follow:

The IUPAC name of mandelic acid is hydroxyl(phenyl)acetic acid.
The molecular formula of pyruvic acid is as follow:

The IUPAC name is 2-Oxopropanoic acid. It is an Alpha keto acid and has no benzene ring.
The structure of citric acid is as follow:

The IUPAC name is 2-hydroxypropane-1,2,3-tricarboxylic acid. Citric acid naturally occurs in various fruits and vegetables. Lemons have considerable high concentration of citric acid. It is also contained in grape food, oranges. Mainly citric acid is used as a flavoring agent and as a preservative in food and beverages specially used in soft drinks and candies.
Hence, the correct option is option A.
Note:
There are various methods of preparation of cinnamic acid. It was first prepared by base catalysed condensation with benzaldehyde and acetone chloride followed by the hydrolysis. It can also be prepared using Knovengel condensation reaction, using benzaldehyde and malonic acid along with some weak base then acid catalysed decarboxylation. It can also be prepared with the help of oxidation of cinnamaldehyde.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




