
2-hexyne gives trans-2-hexene on treatment with:
(A) $Pt/{H_2}$
(B) $Li/N{H_3}$
(C) $Pd/BaS{O_4}$
(D) $LiAl{H_4}$
Answer
577.5k+ views
Hint: 2-hexyne has alkyne as a functional group. Trans isomer is an isomer in which the same groups are on the different side of the carbon-carbon double bond. Trans-2-hexene has an alkene functional group.
Complete step by step solution:
Let’s write the structures of starting material and the product in order to have a better idea about the reagent.
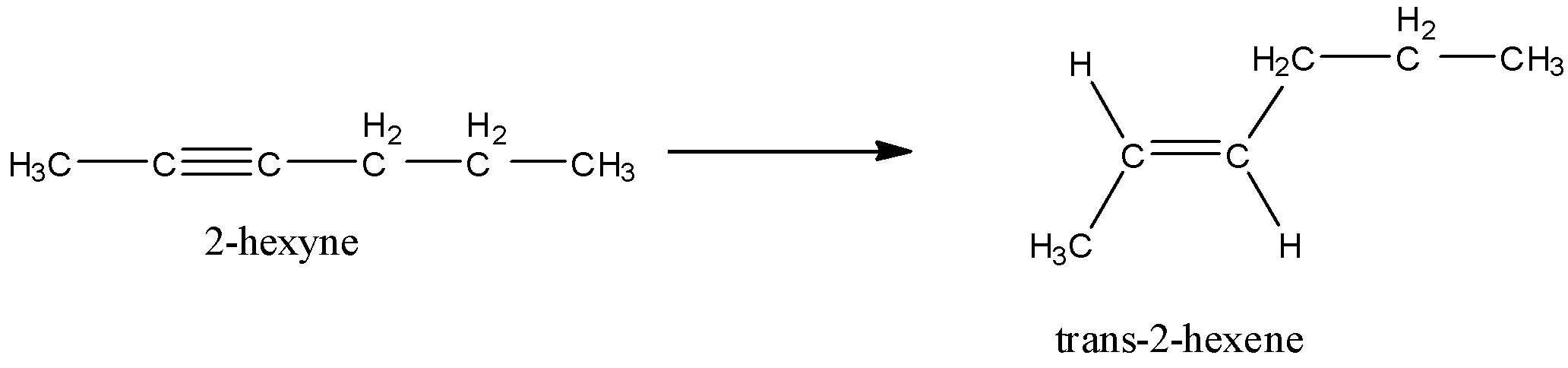
- Here, we can predict from the name 2-hexyne that this compound has an alkyne functional group. This functional group is at 2-position as given in the name.
- Now, the product is having an alkene functional group which can be predicted by –ene suffix. So, basically this reaction is a reduction reaction in which two hydrogen atoms are added across the carbon-carbon triple bond.
- But the main thing is stereochemistry here. The product is trans. That means the like groups are not on the same side of the C-C double bond.
- Hydrogen gas on Platinum metal can reduce the C-C triple bonds to C-C single bonds but we cannot separate the alkene product if it is used in equivalent amounts. So, it is not the correct answer.
- $Li/N{H_3}$ is the reagent that selectively transforms alkyne into trans-alkene. This reaction occurs via a free-radical mechanism. Thus, it is a reduction reaction.
- $Pd/BaS{O_4}$ is also called Lindlar’s catalyst and it reduces alkynes. The specialty of this reagent is that it gives cis-alkenes upon reduction from alkynes.
Thus, we can conclude that the correct answer is (B).
Note: Do not get confused between roles of $Li/N{H_3}$ and $Pd/BaS{O_4}$. Remember that $Li/N{H_3}$ always gives trans alkenes as product while $Pd/BaS{O_4}$ gives cis-alkenes exclusively.
Complete step by step solution:
Let’s write the structures of starting material and the product in order to have a better idea about the reagent.

- Here, we can predict from the name 2-hexyne that this compound has an alkyne functional group. This functional group is at 2-position as given in the name.
- Now, the product is having an alkene functional group which can be predicted by –ene suffix. So, basically this reaction is a reduction reaction in which two hydrogen atoms are added across the carbon-carbon triple bond.
- But the main thing is stereochemistry here. The product is trans. That means the like groups are not on the same side of the C-C double bond.
- Hydrogen gas on Platinum metal can reduce the C-C triple bonds to C-C single bonds but we cannot separate the alkene product if it is used in equivalent amounts. So, it is not the correct answer.
- $Li/N{H_3}$ is the reagent that selectively transforms alkyne into trans-alkene. This reaction occurs via a free-radical mechanism. Thus, it is a reduction reaction.
- $Pd/BaS{O_4}$ is also called Lindlar’s catalyst and it reduces alkynes. The specialty of this reagent is that it gives cis-alkenes upon reduction from alkynes.
Thus, we can conclude that the correct answer is (B).
Note: Do not get confused between roles of $Li/N{H_3}$ and $Pd/BaS{O_4}$. Remember that $Li/N{H_3}$ always gives trans alkenes as product while $Pd/BaS{O_4}$ gives cis-alkenes exclusively.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




