
How many $2{}^\circ $ hydrogens atoms are present in the given compound?
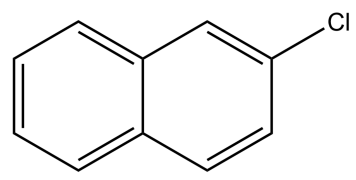
(A) 2
(B) 5
(C) 7
(D) 8

Answer
583.8k+ views
Hint: The carbons attached to one other carbon and having usually 3 hydrogens, in this case, are called primary carbons. The carbon attached to two other carbon is called a secondary carbon. The carbons attached to three other carbons are called a tertiary carbon. The carbons attached to four other carbons are called quaternary carbons.
Complete step by step solution:
-The name for the carbon depends on the number of carbons directly attached to carbon (marked with red), not on the hydrogens.

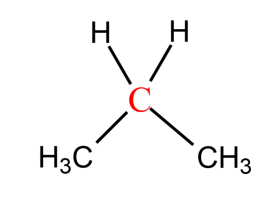
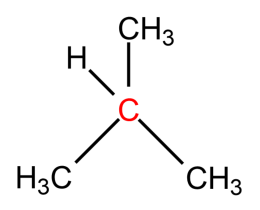
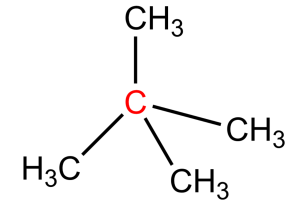
-Same terminology we can apply for hydrogen atoms also. The hydrogens attached to primary carbon are called Primary hydrogens. The hydrogens attached to secondary carbon are called secondary hydrogens. The hydrogens attached to tertiary carbon are called tertiary hydrogen. The hydrogens marked with red colour are primary, the hydrogens marked with green are secondary hydrogen and the hydrogens marked with blue colour are tertiary hydrogens.
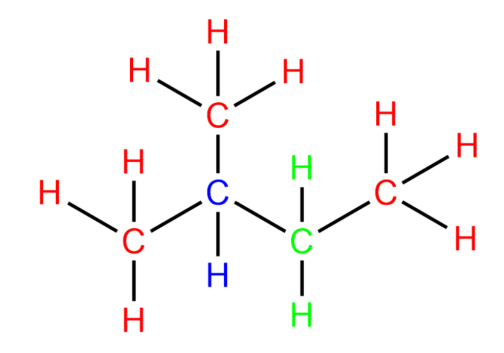
-Based on the above knowledge, let us now try to identify different types of carbon and hydrogen atoms in the molecule given to us.
The carbon and hydrogen atoms marked with green colour are secondary carbons and hydrogens. The carbon and hydrogen marked with blue colour are tertiary carbons and hydrogens.
So, it is clear that we have seven secondary hydrogens in the given molecule.
Hence, the correct answer is option C.
Note: Ortho-substituted is a colourless, oily, aromatic, liquid that may be used to determine the refractive index of crystals by immersion. This compound is toxic and nonpolar which is sometimes used as a powerful biocide and is also known as Basileum. It can occasionally be used as insecticide and fungicide in timber floors of shipping containers. It is also a common solvent for oils, fats, and DDT.
Complete step by step solution:
-The name for the carbon depends on the number of carbons directly attached to carbon (marked with red), not on the hydrogens.




-Same terminology we can apply for hydrogen atoms also. The hydrogens attached to primary carbon are called Primary hydrogens. The hydrogens attached to secondary carbon are called secondary hydrogens. The hydrogens attached to tertiary carbon are called tertiary hydrogen. The hydrogens marked with red colour are primary, the hydrogens marked with green are secondary hydrogen and the hydrogens marked with blue colour are tertiary hydrogens.

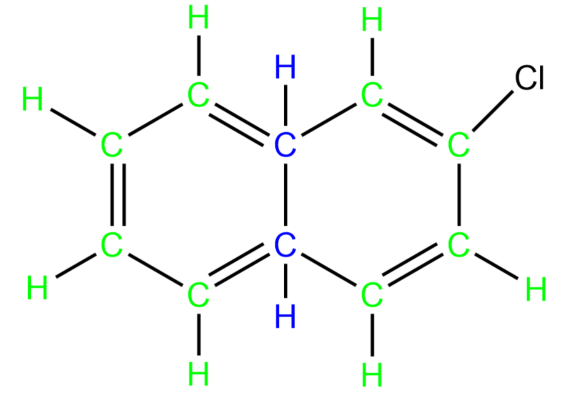
-Based on the above knowledge, let us now try to identify different types of carbon and hydrogen atoms in the molecule given to us.

The carbon and hydrogen atoms marked with green colour are secondary carbons and hydrogens. The carbon and hydrogen marked with blue colour are tertiary carbons and hydrogens.
So, it is clear that we have seven secondary hydrogens in the given molecule.
Hence, the correct answer is option C.
Note: Ortho-substituted is a colourless, oily, aromatic, liquid that may be used to determine the refractive index of crystals by immersion. This compound is toxic and nonpolar which is sometimes used as a powerful biocide and is also known as Basileum. It can occasionally be used as insecticide and fungicide in timber floors of shipping containers. It is also a common solvent for oils, fats, and DDT.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




