
2-Chloro-1,3-butadiene is also known as –
A.Chloroprene
B.Neoprene
C.Isoprene
D.Buna - N
Answer
531.9k+ views
Hint: This question demands information on 2-Chloro-1,3-butadiene. We should know its IUPAC and the structure. It is also known as 2-chlor-1,3 diene, its molecular mass is 88.53g/mol. It is a colourless liquid. Let’s understand more about it.
Complete answer:
The common name of 2-chloro-1,3 butadiene is Chloroprene.
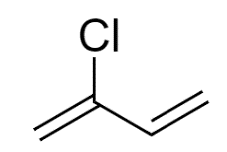
It is a monomer that is used to produce polymer i.e polychloroprene also known as neoprene which is a type of synthetic rubber. Chloroprene is a colorless, volatile liquid. Polymerization should not be done inside the container as it could explode.
The production of chloroprene occurs in three steps-
First, The process of chlorination happens
Second, Isomerization of part of the product stream occur
Third, the Dehydrochlorination of 3,4 dichlorobut-1-ene occurs.
A certain amount of Cl is added to 1,3-butadiene so that we could get a mixture of 3,4-dichlorobut-1-ene and 1,4-dichlorobut-2-ene. Then the isomerization of 1,4-dichloro occurs to form 3,4 isomers, It is treated with a base so that dehydrochlorination could be done to 2-chlorobuta-1,3-diene. Due to dehydrohalogenation loss of H atom occurs at 3 position and loss of Cl atom occurs at 4 position. It results in the formation of a double bond between carbon 3 and 4.
So, 2-Chloro-1,3-butadiene is also known as Option(A) chloroprene.
Note:
Chloroprene could be toxic, If someone gets exposed to a high concentration of it, They could face issues like headaches, dizziness, fatigue, loss of sleep, respiratory problems, nausea, gastrointestinal issues, hair loss, conjunctivitis, chest pain, cancer, dermatitis, liver function problems, the immune system could become weak. In animals, there could be tumors in multiple organs.
Complete answer:
The common name of 2-chloro-1,3 butadiene is Chloroprene.

It is a monomer that is used to produce polymer i.e polychloroprene also known as neoprene which is a type of synthetic rubber. Chloroprene is a colorless, volatile liquid. Polymerization should not be done inside the container as it could explode.
The production of chloroprene occurs in three steps-
First, The process of chlorination happens
Second, Isomerization of part of the product stream occur
Third, the Dehydrochlorination of 3,4 dichlorobut-1-ene occurs.
A certain amount of Cl is added to 1,3-butadiene so that we could get a mixture of 3,4-dichlorobut-1-ene and 1,4-dichlorobut-2-ene. Then the isomerization of 1,4-dichloro occurs to form 3,4 isomers, It is treated with a base so that dehydrochlorination could be done to 2-chlorobuta-1,3-diene. Due to dehydrohalogenation loss of H atom occurs at 3 position and loss of Cl atom occurs at 4 position. It results in the formation of a double bond between carbon 3 and 4.
So, 2-Chloro-1,3-butadiene is also known as Option(A) chloroprene.
Note:
Chloroprene could be toxic, If someone gets exposed to a high concentration of it, They could face issues like headaches, dizziness, fatigue, loss of sleep, respiratory problems, nausea, gastrointestinal issues, hair loss, conjunctivitis, chest pain, cancer, dermatitis, liver function problems, the immune system could become weak. In animals, there could be tumors in multiple organs.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




