
2-butanol is converted into 2-methylbutanoic acid by:
A. (i) Cu (ii) HCN (iii) ${{H}_{3}}{{O}^{+}}$
B. (i) HCN (ii) ${{H}_{3}}{{O}^{+}}$
C. (i) $PC{{l}_{5}}$ (ii) KCN (iii) ${{H}_{3}}{{O}^{+}}$
D. (i) KCN (ii) ${{H}_{3}}{{O}^{+}}$
Answer
528.3k+ views
Hint: Butanol is a molecule containing 4 carbon atoms along with an alcoholic group that’s why the suffix ol is attached with it. While butanoic acid is those compounds in which an acidic group is present.
Complete answer:
Butanol is primarily used as a solvent and as an intermediate in chemical synthesis and also used as a fuel. 2-butanol describes the position of alcoholic group i.e. OH group is present on 2nd carbon atom which can be shown as:

2-methylbutanoic acid tells that a 2nd carbon methyl group is attached and an acidic group is present on the first position. It exists in two enantiomeric forms which are defined as (R) and (S)-2-methylbutanoic acid. (R)-2-methylbutanoic acid occurs naturally in cocoa beans and (S)-2-methylbutanoic is present in many fruits such as apples and apricots.
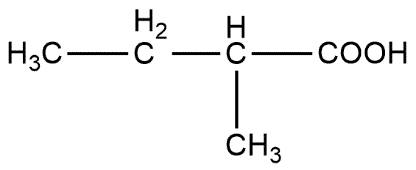
2-butanol is converted into 2-methylbutanoic acid step by step:
Ist step converts 2-butanol to 2-chlorobutane
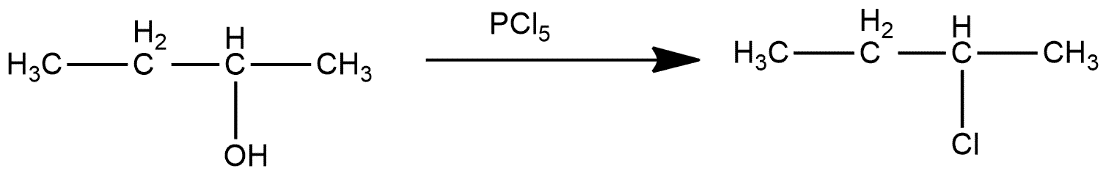
In second step, 2-chlorobutane reacts with KCN and get Cl group get replaced with CN group which can be shown as:
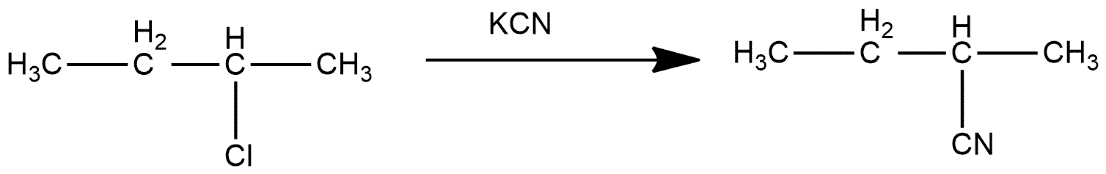
In third step, it reacts with ${{H}_{3}}{{O}^{+}}$and get converted into 2-methylbutanoic acid which can be shown as:

Thus we can say that option C is the correct answer.
Note:
2-methylbutanoic acid is a slightly volatile, colorless liquid with a pungent cheesy odor. The smell differs significantly between the two enantiomeric forms i.e. (S)-2-Methylbutyric acid has a pleasantly sweet, fruity odor while (R)-2-methylbutanoic acid has a cheesy, sweaty odor. The main use of the materials and their esters used as flavours and food additives.
Complete answer:
Butanol is primarily used as a solvent and as an intermediate in chemical synthesis and also used as a fuel. 2-butanol describes the position of alcoholic group i.e. OH group is present on 2nd carbon atom which can be shown as:

2-methylbutanoic acid tells that a 2nd carbon methyl group is attached and an acidic group is present on the first position. It exists in two enantiomeric forms which are defined as (R) and (S)-2-methylbutanoic acid. (R)-2-methylbutanoic acid occurs naturally in cocoa beans and (S)-2-methylbutanoic is present in many fruits such as apples and apricots.

2-butanol is converted into 2-methylbutanoic acid step by step:
Ist step converts 2-butanol to 2-chlorobutane

In second step, 2-chlorobutane reacts with KCN and get Cl group get replaced with CN group which can be shown as:

In third step, it reacts with ${{H}_{3}}{{O}^{+}}$and get converted into 2-methylbutanoic acid which can be shown as:

Thus we can say that option C is the correct answer.
Note:
2-methylbutanoic acid is a slightly volatile, colorless liquid with a pungent cheesy odor. The smell differs significantly between the two enantiomeric forms i.e. (S)-2-Methylbutyric acid has a pleasantly sweet, fruity odor while (R)-2-methylbutanoic acid has a cheesy, sweaty odor. The main use of the materials and their esters used as flavours and food additives.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




