
2,3-dimethyl-2-butene can be prepared by heating which of the following compounds with a strong acid?
A. ${{\left( C{{H}_{3}} \right)}_{2}}C=CH-C{{H}_{2}}-C{{H}_{3}}$
B. ${{\left( C{{H}_{3}} \right)}_{2}}CH-C{{H}_{2}}-CH=C{{H}_{2}}$
C.
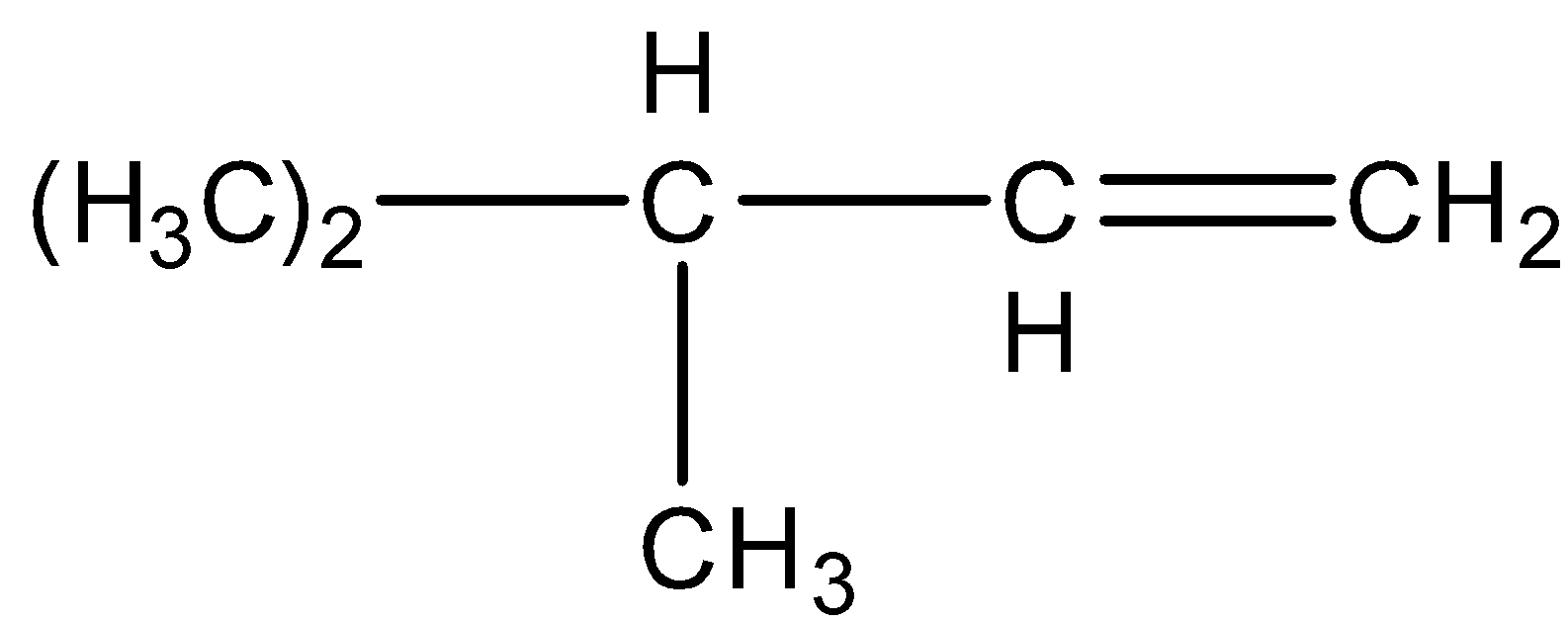
D. ${{\left( C{{H}_{3}} \right)}_{3}}CH-CH=C{{H}_{2}}$

Answer
579.6k+ views
Hint: Strong acids liberate ${{H}^{+}}$ ions and so are used in organic chemistry for protonation. We can check for the formation of the desired product by heating these compounds. Protonation results in the formation of a carbocation which has the tendency to rearrange itself and we need to find the product accordingly.
Complete answer:
-Acids are the compounds that donate ${{H}^{+}}$ion to become anion. Strong acids can liberate these ions at a very fast rate while weak acids liberate these ions very slowly. When these acids are made to react with a multiple bond, they lead to protonation which results in the formation of a carbocation.
-Carbocation is a reaction intermediate and it is converted into some compound before the completion of the reaction due to its high instability. They act as lewis acid and have 6 valence electrons.
-Carbocations can be stabilized by the positive effects like positive mesomeric effect, hyperconjugation and positive inductive effect. So they are more stable if the degree of carbon is more.
-As the carbocations can be stabilized by the effects, they can be rearranged within the compound and this leads to the formation of a specific compound on protonation. No other compound can be formed due to the carbocation stability.
-In the question, we see that we need to heat a compound by an acid which means protonation takes place. The compound that will form 2,3-dimethyl-2-butene on heating with a strong acid is ${{\left( C{{H}_{3}} \right)}_{3}}CH-CH=C{{H}_{2}}$.
-The proton will be added in the first step leading to the formation of the carbocation. This carbocation will then rearrange itself to gain stability by shifting the methyl group as it has +I effect. Then deprotonation will result in the formation of the desired akene. The steps can be shown as
$\begin{align}
& {{\left( C{{H}_{3}} \right)}_{3}}CH-CH=C{{H}_{2}}\xrightarrow[protonation]{Strong\text{ }acid}{{\left( C{{H}_{3}} \right)}_{3}}C-C{{H}^{+}}-C{{H}_{3}} \\
& {{\left( C{{H}_{3}} \right)}_{3}}C-C{{H}^{+}}-C{{H}_{3}}\xrightarrow{rearrangement}{{\left( C{{H}_{3}} \right)}_{2}}{{C}^{+}}-C\left( C{{H}_{3}} \right)H-C{{H}_{3}} \\
& {{\left( C{{H}_{3}} \right)}_{2}}{{C}^{+}}-C\left( C{{H}_{3}} \right)H-C{{H}_{3}}\xrightarrow{deprotonation(-{{H}^{+}})}C{{H}_{3}}C\left( C{{H}_{3}} \right)=C\left( C{{H}_{3}} \right)C{{H}_{3}} \\
& \\
\end{align}$
Thus the correct option is D.
Note:
Carbocations can be formed by many reactions like hydration of alcohols, from alkyl halides, from alkenes,from aldehydes and ketones and many other compounds. Whenever formation of carbocation occurs, always keep in mind that rearrangement should be done before forming the products.
Complete answer:
-Acids are the compounds that donate ${{H}^{+}}$ion to become anion. Strong acids can liberate these ions at a very fast rate while weak acids liberate these ions very slowly. When these acids are made to react with a multiple bond, they lead to protonation which results in the formation of a carbocation.
-Carbocation is a reaction intermediate and it is converted into some compound before the completion of the reaction due to its high instability. They act as lewis acid and have 6 valence electrons.
-Carbocations can be stabilized by the positive effects like positive mesomeric effect, hyperconjugation and positive inductive effect. So they are more stable if the degree of carbon is more.
-As the carbocations can be stabilized by the effects, they can be rearranged within the compound and this leads to the formation of a specific compound on protonation. No other compound can be formed due to the carbocation stability.
-In the question, we see that we need to heat a compound by an acid which means protonation takes place. The compound that will form 2,3-dimethyl-2-butene on heating with a strong acid is ${{\left( C{{H}_{3}} \right)}_{3}}CH-CH=C{{H}_{2}}$.
-The proton will be added in the first step leading to the formation of the carbocation. This carbocation will then rearrange itself to gain stability by shifting the methyl group as it has +I effect. Then deprotonation will result in the formation of the desired akene. The steps can be shown as
$\begin{align}
& {{\left( C{{H}_{3}} \right)}_{3}}CH-CH=C{{H}_{2}}\xrightarrow[protonation]{Strong\text{ }acid}{{\left( C{{H}_{3}} \right)}_{3}}C-C{{H}^{+}}-C{{H}_{3}} \\
& {{\left( C{{H}_{3}} \right)}_{3}}C-C{{H}^{+}}-C{{H}_{3}}\xrightarrow{rearrangement}{{\left( C{{H}_{3}} \right)}_{2}}{{C}^{+}}-C\left( C{{H}_{3}} \right)H-C{{H}_{3}} \\
& {{\left( C{{H}_{3}} \right)}_{2}}{{C}^{+}}-C\left( C{{H}_{3}} \right)H-C{{H}_{3}}\xrightarrow{deprotonation(-{{H}^{+}})}C{{H}_{3}}C\left( C{{H}_{3}} \right)=C\left( C{{H}_{3}} \right)C{{H}_{3}} \\
& \\
\end{align}$
Thus the correct option is D.
Note:
Carbocations can be formed by many reactions like hydration of alcohols, from alkyl halides, from alkenes,from aldehydes and ketones and many other compounds. Whenever formation of carbocation occurs, always keep in mind that rearrangement should be done before forming the products.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




