
(1) What is the formula of plaster of paris.
(2) What are disproportionation reactions? Give examples.
Answer
573.3k+ views
Hint: Plaster of Paris is prepared by heating Gypsum which is a sulphate mineral made up of calcium sulphate dihydrate. Disproportionation reactions are basically redox reactions where oxidation and reduction both take place simultaneously.
Complete answer:
(1) As we all know that Plaster of Paris is named because of its preparation by heating Gypsum which is mainly found in Paris. Gypsum is basically a sulphate mineral which is composed of calcium sulphate dihydrate and has the chemical formula as $CaS{O_4}.2{H_2}O$. It is heated at a temperature of ${120^ \circ }C$ to produce calcium sulphate hemihydrate commonly known as the Plaster of Paris. This can be explained using the chemical equation:
$CaS{O_4}.2{H_2}O \to CaS{O_4}.\dfrac{1}{2}{H_2}O + \dfrac{3}{2}{H_2}O$
Therefore we can say that the chemical formula for Plaster of Paris is $(CaS{O_4}).\dfrac{1}{2}{H_2}O$or $2CaS{O_4}.{H_2}O$.
(2) We have learnt that Disproportionation reactions are the redox reactions which include both oxidation and reduction of atoms of the same element simultaneously. The reacting species must possess an element which has at the very least three oxidation states for the redox reactions to occur. The higher and lower oxidation states are available for oxidation and reduction to occur while the element in the reacting species is present in the intermediate oxidation state.
For example: the decomposition of hydrogen peroxide is a common disproportionation reaction where oxygen atoms undergo oxidation as well as reduction. We can represent this in a chemical equation as:
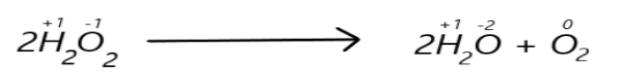
Here we can see that the oxidation number of oxygen which is $ - 1$ in hydrogen peroxide and it decreases to $ - 2$in water and increases to $0$in oxygen atom.
Note: Plaster of Paris hardens when it is moistened and allowed to dry and is widely used as a protective coating on the walls and ceilings of the buildings. It is also used as an agent for moulding and casting. Skin infections and minor cuts can be prevented by using Hydrogen peroxide as an antiseptic.
Complete answer:
(1) As we all know that Plaster of Paris is named because of its preparation by heating Gypsum which is mainly found in Paris. Gypsum is basically a sulphate mineral which is composed of calcium sulphate dihydrate and has the chemical formula as $CaS{O_4}.2{H_2}O$. It is heated at a temperature of ${120^ \circ }C$ to produce calcium sulphate hemihydrate commonly known as the Plaster of Paris. This can be explained using the chemical equation:
$CaS{O_4}.2{H_2}O \to CaS{O_4}.\dfrac{1}{2}{H_2}O + \dfrac{3}{2}{H_2}O$
Therefore we can say that the chemical formula for Plaster of Paris is $(CaS{O_4}).\dfrac{1}{2}{H_2}O$or $2CaS{O_4}.{H_2}O$.
(2) We have learnt that Disproportionation reactions are the redox reactions which include both oxidation and reduction of atoms of the same element simultaneously. The reacting species must possess an element which has at the very least three oxidation states for the redox reactions to occur. The higher and lower oxidation states are available for oxidation and reduction to occur while the element in the reacting species is present in the intermediate oxidation state.
For example: the decomposition of hydrogen peroxide is a common disproportionation reaction where oxygen atoms undergo oxidation as well as reduction. We can represent this in a chemical equation as:

Here we can see that the oxidation number of oxygen which is $ - 1$ in hydrogen peroxide and it decreases to $ - 2$in water and increases to $0$in oxygen atom.
Note: Plaster of Paris hardens when it is moistened and allowed to dry and is widely used as a protective coating on the walls and ceilings of the buildings. It is also used as an agent for moulding and casting. Skin infections and minor cuts can be prevented by using Hydrogen peroxide as an antiseptic.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




