
1-
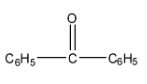
2-
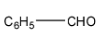
3-
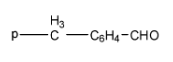
4-
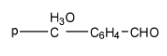
Correct order for Nucleophilic addition reaction.
(A)- 2 > 1 > 3 > 4
(B)- 4 > 3 > 2 > 1
(C)- 2 > 3 > 4 > 1
(D)- 4 > 2 > 3 > 1

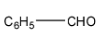


Answer
523.4k+ views
Hint: Reactivity of aldehydes towards Nucleophilic addition reaction is more than the reactivity of ketones. And the reactivity of the carbonyl group can be decided on the factors of the inductive effect and resonance effect.
Complete step by step solution:
All the compounds given above have a carbonyl group. There are two functional groups that have carbonyl groups, they are aldehydes and ketones.
The reactivity of aldehydes towards a Nucleophilic addition reaction is more than the reactivity of ketones, or we can say that ketones are less reactive than aldehydes. And the reactivity of the carbonyl group can be decided on the factors of the inductive effect and resonance effect.
The displacement of $\sigma -electrons$ along the saturated carbon chain whenever an electron-withdrawing or electron-donating group is present at the end of the carbon chain is called inductive effect or I-effect.
–I-Effect is caused when the substituent attached to the end of the carbon chain is electron-withdrawing.
+I-Effect is when the substituent attached to the end of the carbon chain is electron-donating.
The flow of electrons from one part of the conjugated system to the other creating centers of low and high electron density due to the phenomenon of resonance is called resonance effect or R-effect.
Groups that donate electrons to the double bond are said to have a +R-effect.
Groups which withdraw electrons from the double bond are said to have –R-effect
Both the –I-Effect and –R-Effect increases the reactivity of the carbonyl group towards nucleophilic addition reaction.
But the substituents like $-OC{{H}_{3}}$ and $-C{{H}_{3}}$ groups have both +I-Effect and +R-Effect, and this decreases the reactivity of the carbonyl group.
So the order will be: 2 > 3 > 4 > 1
Therefore, the correct answer is an option (C)- 2 > 3 > 4 > 1.
Note: The carbonyl group shows a Nucleophilic addition reaction because the carbonyl group is a polar group and the oxygen being more electronegative atom acquires a slight negative charge and behaves as an electrophile.
Complete step by step solution:
All the compounds given above have a carbonyl group. There are two functional groups that have carbonyl groups, they are aldehydes and ketones.
The reactivity of aldehydes towards a Nucleophilic addition reaction is more than the reactivity of ketones, or we can say that ketones are less reactive than aldehydes. And the reactivity of the carbonyl group can be decided on the factors of the inductive effect and resonance effect.
The displacement of $\sigma -electrons$ along the saturated carbon chain whenever an electron-withdrawing or electron-donating group is present at the end of the carbon chain is called inductive effect or I-effect.
–I-Effect is caused when the substituent attached to the end of the carbon chain is electron-withdrawing.
+I-Effect is when the substituent attached to the end of the carbon chain is electron-donating.
The flow of electrons from one part of the conjugated system to the other creating centers of low and high electron density due to the phenomenon of resonance is called resonance effect or R-effect.
Groups that donate electrons to the double bond are said to have a +R-effect.
Groups which withdraw electrons from the double bond are said to have –R-effect
Both the –I-Effect and –R-Effect increases the reactivity of the carbonyl group towards nucleophilic addition reaction.
But the substituents like $-OC{{H}_{3}}$ and $-C{{H}_{3}}$ groups have both +I-Effect and +R-Effect, and this decreases the reactivity of the carbonyl group.
So the order will be: 2 > 3 > 4 > 1
Therefore, the correct answer is an option (C)- 2 > 3 > 4 > 1.
Note: The carbonyl group shows a Nucleophilic addition reaction because the carbonyl group is a polar group and the oxygen being more electronegative atom acquires a slight negative charge and behaves as an electrophile.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




